Calculate the atomic mass for magnesium given the following data for its natural isotopes. 24 Mg: 25Mg:
Question:
Calculate the atomic mass for magnesium given the following data for its natural isotopes.
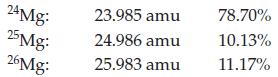
Transcribed Image Text:
24 Mg: 25Mg: 26Mg: 23.985 amu 24.986 amu 25.983 amu 78.70% 10.13% 11.17%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the atomic mass for magnesium given the following data for its natural isoto...View the full answer

Answered By

Poonam Chaudhary
I have 15 month+ Teaching Experience
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Modify the code to add another attribute and a method that fits (is consistent with) the object the class is modeling, or suggest a different version of the code. Write the main method to demonstrate...
-
Calculate the atomic mass for zinc given the following data for its natural isotopes. 64Zn 66Zn 67Zn 68 Zn 70Zn 63.929 amu 65.926 amu 66.927 amu 67.925 amu 69.925 amu 48.89% 27.81% 4.11% 18.57% 0.62%
-
Calculate the atomic mass for iron given the following data for its natural isotopes. 54Fe: 56Fe: 57 Fe: 58 Fe: 53.940 amu 55.935 amu 56.935 amu 57.933 amu 5.82% 91.66% 2.19% 0.33%
-
In Exercises 1318, find the average rate of change of the function from x 1 to x 2 . f(x) = 3x from x 0 to x = 5
-
How is operating leverage calculated?
-
Gray encoding of decimal numbers ensures that only one bit changes when the decimal number changes by one unit. Let b 1 b 2 b 3 ... b n represent an ordinary bi nary representation of a decimal...
-
Listen-and-look study. Where do you look when you are listening to someone speak? Researchers have discovered that listeners tend to gaze at the eyes or mouth of the speaker. In a study published in...
-
A New Yorker travels to New Jersey to buy a $100 telephone answering machine. The New Jersey Company that sells the machine then deposits the $100 check in its account at a New York bank. How would...
-
Bonita's Manufacturing Company can make 1 0 0 units of a necessary component part with the following costs: If Bonita's Manufacturing Company can purchase the component externally for $ 2 2 0 0 0 0...
-
Which has the longer wavelength: red light or yellow light?
-
Calculate the atomic mass for lithium given the following data for its natural isotopes. 'Li: 7Li: 6.015 amu 7.016 amu 7.42% 92.58%
-
Shiroma, Inc., is a pork processor. Its plants, located in the Midwest, produce several products from a common process: sirloin roasts, chops, spare ribs, and the residual. The roasts, chops, and...
-
Verify the results of Eq. (14.48) for the properties of the chiral projection operators. Data from Eq. 14.48 P = P+ P+ + P = 1 P_P+ P+P = 0 Py" = y P
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
a. Draw all possible stereoisomers for the following compound b. Which isomers are optically inactive (will not rotate plane-polarized light)? HOCH,CH-CH CHCH OH OH OH OH
-
Swifty company is a publicly held corporation whose $1 par value stock is actively traded at $30 per share. The company issued 3400 shares of stock to acquire land recently advertised at $93000. When...
-
What is meant by going private? What are some advantages and disadvantages?
-
How do companies manage the maturity structure of their debt? MINI CASE Randys, a family-owned restaurant chain operating in Alabama, has grown to the point where expansion throughout the entire...
-
Under what conditions would a firm exercise a bonds call provision? MINI CASE Randys, a family-owned restaurant chain operating in Alabama, has grown to the point where expansion throughout the...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


