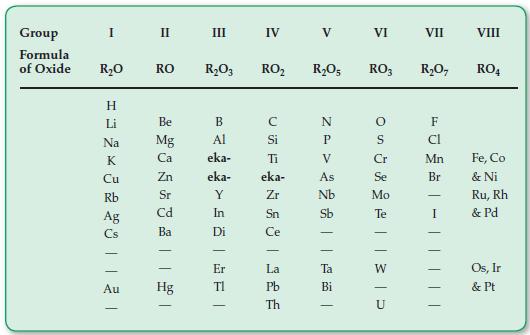
Examine Figure 5.1 and determine the name of the element that Mendeleev predicted before its discovery and
Question:
Examine Figure 5.1 and determine the name of the element that Mendeleev predicted before its discovery and called ekaaluminum.
Figure 5.1
Transcribed Image Text:
Group Formula of Oxide I R₂0 = = 2 × 3 2 2011 21 Cu Ag Au II RO Be Mg Ca Zn Sr Cd Ba Hg III R₂O3 RO₂ B Al eka- eka- Y In Di - Er TI IV - CSF с Si Ti eka- Zr Sn Ce - La Pb Th V R₂O5 RO3 N P V As Nb Sb Ta Bi VI - OS 35 Cr Se Mo Te VII R₂O7 FU Mn Br I |||| VIII RO4 Fe, Co & Ni Ru, Rh & Pd Os, Ir & Pt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The element that Mendeleev predicted before its discovery and called ekaaluminum is gallium ...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Examine Figure 5.1 and determine the name of the element that Mendeleev predicted before its discovery and called ekaboron. Figure 5.1 Group Formula of Oxide I R0 = 32 3 2 2011 21 Cu Ag Au II RO Be...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
A 300-cm-long piece of straight wire is aligned vertically along the z-axis. The wire carries a downward current of 6.00 A. There is a 2.00-T magnetic field in the negative x-direction surrounding...
-
General Long-term Liability and Capital Asset Transactions The Village of Nassau issued a 3-year, 6 percent note in the amount of $100,000 to finance the purchase of vehicles for the Public Works...
-
You are going to pressurize a tube using a mechanical pump. The standard bicycle tube is wrapped around a 24-in. diameter metal rim. The inflow to the pump is constant at 1 ft 3 /min. The density of...
-
Baseball The number of games played in the World Series from 1903 to 2009 (Source: Major League Baseball). Games played 4 5 6 7 8 Frequency 20 23 23 36 3
-
The following information pertains to VI Division, which has $1,400,000 in investments. Division sales revenue $900,000 Less division expenses 480,000 Division income $420,000 The companys cost of...
-
This year, FGH Partnership generated $800,000 ordinary business income. FGH has two equal partners: Triad LLC and Beta, an S corporation. Triad LLC has three members: Mr. T, who owns a 40 percent...
-
Explain why the ionization energy for the alkali metals is less than the ionization energy for the alkaline earth metals.
-
Predict two ionic charges for hydrogen. Write the formulas of the two ions and explain the ionic charges.
-
A salesman wants to visit four California cities, San Francisco, Sacramento, San Jose, and Fresno. Driving distances are shown in Figure 9.6. What is the shortest trip starting and ending in San...
-
After further negotiation Roger and Benedicta agreed to use standard preferred stock after all. In her counter-offer, however, Benedicta has proposed that her shares pay cumulative non-cash dividends...
-
Use the Empirical Rule to answer the questions below: The distribution of weights for newborn babies is approximately normally distributed with a mean of 7.5 pounds and a standard deviation of 0.6...
-
Assessment Coversheet Unit/s assessed FNSTPB503 Apply Legal Principles in Contract and Consumer Law Assessment name FNSTPB503 Assignment Type of Assessment This summative assessment will enable your...
-
Question 1 (35 Points) A soil profile is provided below. The estimated stresses from a spread footing overlying a 10 meter thick normally consolidated clay layer are: 180.0 kN/sq m (top of the clay...
-
Which of the following molecules would you expect to have a dipole moment of zero? To answer parts g and h, you may need to consult your answers to Problem 23 a and b. a. CH3CH3 b. H2C==O c. CH2Cl2...
-
What are the main distinctions between the different schools of legal interpretation?
-
Archer Inc. issued $4,000,000 par value, 7% convertible bonds at 99 for cash. If the bonds had not included the conversion feature, they would have sold for 95. Prepare the journal entry to record...
-
Petrenko Corporation has outstanding 2,000 $1,000 bonds, each convertible into 50 shares of $10 par value common stock. The bonds are converted on December 31, 2010, when the unamortized discount is...
-
Pechstein Corporation issued 2,000 shares of $10 par value common stock upon conversion of 1,000 shares of $50 par value preferred stock. The preferred stock was originally issued at $60 per share....
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


