Explain the decrease in solubility for the following acids in water. Acid CH3COOH acetic acid, pentanoic acid,
Question:
Explain the decrease in solubility for the following acids in water.
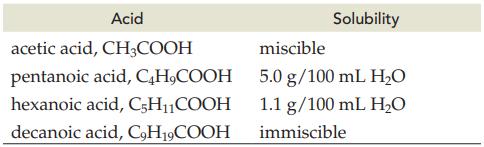
Transcribed Image Text:
Acid CH3COOH acetic acid, pentanoic acid, C4H₂COOH hexanoic acid, C5H₁₁COOH decanoic acid, C9H19COOH Solubility miscible 5.0 g/100 mL H₂O 1.1 g/100 mL H₂O immiscible
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The decrease in solubility observed for the given acids in water can be explained by several factors including molecular structure polarity and interm...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Explain the decrease in solubility for the following alcohols in water. Alcohol ethanol, CH5OH pentanol, C5H1OH hexanol, C6H3OH decanol, C0H21OH Solubility miscible 2.2 g/100 mL HO 0.59 g/100 mL HO...
-
Various amino acids have utility as food additives and in medical applications. They are often synthesized by fermentation using a specific microorganism to convert a substrate (e.g., a sugar) into...
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
A potential difference of 1.20 V will be applied to a 33.0 m length of 18-gauge copper wire (diameter = 0.0400 in.). Calculate (a) The current, (b) The magnitude of the current density, (c) The...
-
Pay Corporation acquired a 75 percent interest in Sue Corporation for $600,000 on January 1, 2011, when Sue's equity consisted of $300,000 capital stock and $100,000 retained earnings. The fair...
-
Leonardos filing status is head of household. He has modified Adjusted Gross Income of $33,000, and he made a $3,000 contribution to his IRA. What is the amount of his retirement savings...
-
Ascorbic acid reduces goat stress. Refer to the Animal Science Journal (May 2014) study on the use of ascorbic acid (AA) to reduce the stress in goats during transportation, Exercise 10.7 (p. 537)....
-
Your firm needs to raise $100 million in funds. You can borrow short term at a spread of 1% over LIBOR. Alternatively, you can issue 10-year, fixed-rate bonds at a spread of 2.50% over 10-year...
-
Having too much inventory can cost the company money in all the following areas except O storage costs. O interest costs. high tech goods becoming obsolete. O depreciation
-
Identify the solutes and solvents in the following solutions. (a) 80-proof ethyl alcohol (40% ethanol in water) (b) 190-proof ethyl alcohol (95% ethanol in water).
-
Phenol, C 6 H 5 OH, is 7% soluble in water. Explain why phenol is only partially soluble even though molecules contain an electronegative oxygen atom.
-
Compute and use traditional allocation rate (Learning Objective 1) Refer to the Mission Data Set. 1. Compute Missions indirect cost allocation rate. 2. Compute the total costs assigned to the Webb...
-
Gilbert Canned Produce (GCP) packs and sells three varieties of canned produce: green beans; sweet peas; and tomatoes. The company is currently operating at 82 percent of capacity. Worried about the...
-
Apply at least two of the theories (of your choice) to your personal experience? The theories are Leader-Member Exchange Theory (LMX Model), the Situational Leadership Model, the Contingency Model...
-
Game theory is used in economics, social science and computer science to understand and predict the behaviour of people and intelligent entities. In project management and business scenarios, it can...
-
During a chemistry lab, you take a 0.2 kg sample of ice and put it in a beaker with a thermometer. You then place the beaker with the ice on a hot plate, and turn on the hot plate. This hot plate...
-
Selected information from Carla Vista Ltd.'s statement of financial position and statement of income is as follows: Carla Vista Ltd. Statement of Financial Position (partial) December 31 2024 2023...
-
Why are bank credit card sales similar to cash sales for a business?
-
Open Text Corporation provides a suite of business information software products. Exhibit 10-9 contains Note 10 from the companys 2013 annual report detailing long-term debt. Required: a. Open Text...
-
Calculating Annuity Values Dinero Bank offers you a $30,000, seven-year term loan at 8 percent annual interest. What will your annual loan payment be?
-
Calculating Perpetuity Values The Maybe Pay Life Insurance Co. is trying to sell you an investment policy that will pay you and your heirs $20,000 per year forever. If the required return on this...
-
Calculating Perpetuity Values In the previous problem, suppose a sales associate told you the policy costs $280,000. At what interest rate would this be a fair deal?
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


