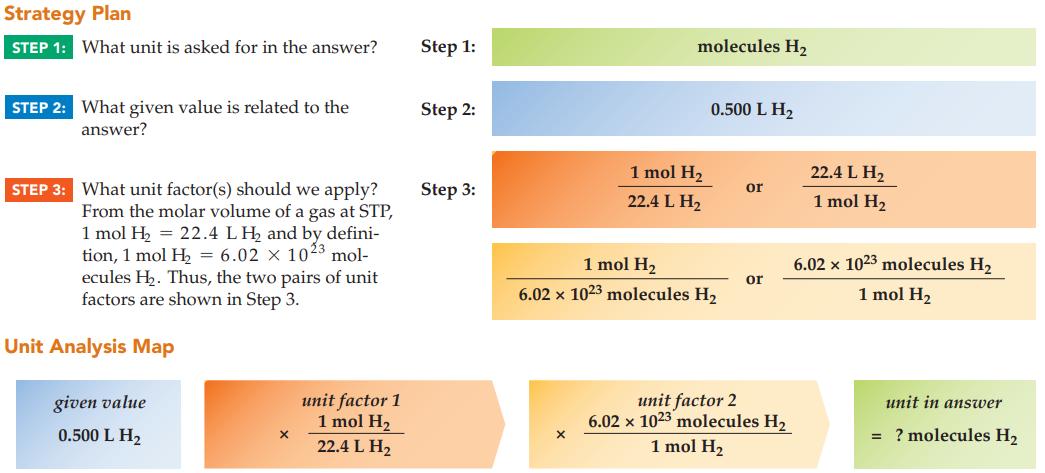
How many molecules of hydrogen gas, H 2 , occupy 0.500 L at STP? Strategy Plan STEP
Question:
How many molecules of hydrogen gas, H2, occupy 0.500 L at STP?
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? From the molar volume of a gas at STP, 1 mol H₂ = 22.4 LH₂ and by defini- tion, 1 mol H₂ = 6.02 x 1023 mol- ecules H₂. Thus, the two pairs of unit factors are shown in Step 3. Unit Analysis Map given value 0.500 L H₂ X unit factor 1 1 mol H₂ 22.4 L H₂ Step 1: Step 2: Step 3: molecules H₂ X 1 mol H₂ 22.4 L H₂ 0.500 L H₂ 1 mol H₂ 6.02 x 1023 molecules H₂ or or unit factor 2 6.02 x 1023 molecules H₂ 1 mol H₂ 22.4 L H₂ 1 mol H₂ 6.02 x 1023 molecules H₂ 1 mol H₂ unit in answer = ? molecules H₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
We apply the unit factor 1 mol H224 LH t...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Strong and Tall's Inc weight gain program headline says, "Gain up to 20 kg in 60 days or we will give you your money back." The 2 types of headline this ad shows is: a. command & benefit b. command &...
-
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia.? N2(g)+3H2(g)---->2NH3(g) Assume 4 molecules of nitrogen and 9 molecules of hydrogen are...
-
How many molecules of H2 are needed to react with 29 molecules of N2 to make ammonia if the balanced chemical equation is N2 + 3H2 2NH3?
-
Write the C++ code for a function that receives an integer, a double number, and the address of a double variable from the calling statement. The function should multiply the integer by the double...
-
Brasswood Corporation reports the following amounts in its 2012 financial statements: Instructions(a) Compute the December 31, 2012, balance in stockholders?? equity.(b) Compute the debt to total...
-
Test the claim about the difference between two population means 1 and 2 at the level of significance a. Assume the samples are random and independent, and the populations are normally distributed....
-
Calculating the Monthly Housing Payment. Ben and Carla Manchester plan to buy a condominium. They will obtain a $150,000, 30-year mortgage at 6 percent. Their annual property taxes are expected to be...
-
(a) Using the law of cosines, show that Eq. 3.17 can be written as follows where r and are the usual spherical polar coordinates, with the z axis along the line through q. In this form it is obvious...
-
Bolivia Corp. has outstanding accounts receivable totaling $2.46 million as of December 31 and sales on credit during the year of $8.2 million. There is also a debit balance of $14,000 in the...
-
Given Avogadros number of shotput balls, which of the following is the best estimate of the total mass: an automobile, the Empire State building, or Earth?
-
How many molecules of ozone are in one mole of gas?
-
What initial speed would a particle need to be given at the surface of Earth if it is to have a final speed that is equal to its escape speed when it is very far from Earth? Neglect any effects due...
-
Jasmine Minoza, the chief information officer of a Canada- based designer of video games, Adventure Gaming, Inc. (AGI), is considering outsourcing her companys software development activities to...
-
The input to the circuit of Fig. 5-23 with RC = 1 is v 1 = sin t. Write KCL at node B and solve for v 2 . +1 VI R B A + C D 3+ 10-41. 12
-
Draw an angle of 120. First draw a straight line about 6cm long. Place the protractor on the line so that the central cross hair is on one of the end points of the line. Make sure the line lines up...
-
Draw a seriesparallel switch circuit that implements the function f(x, y, z) = 1 if inputs xyz represent either 1 or a prime number in binary (xyz = 001, 010, 011, 101, 111).
-
A parallel-plate capacitor connected to a battery maintaining a potential difference \(V\) across the capacitor initially stores electric potential energy \(U_{1}^{E}\). If the plate area is doubled...
-
The associated Legendre functions Pl|m| (w) are defined by Verify that P00(w) = 1, P01(w) = w, P11(w) = (1 - w2)1/2 and find P02(w), P12(w), and P22(w). It can be shown that (Pauling and Wilson, page...
-
Dan and Diana file a joint return. Dan earned $31,000 during the year before losing his job. Diana received Social Security benefits of $5,000. a. Determine the taxable portion of the Social Security...
-
Gross Profit Method Castlevania Company lost most of its inventory in a fire in December just before the year-end physical inventory was taken. The corporations books disclosed the following....
-
Gross Profit Method You are called by Kevin Garnett of Celtic Co. on July 16 and asked to prepare a claim for insurance as a result of a theft that took place the night before. You suggest that an...
-
Gross Profit Method Sliver Lumber Company handles three principal lines of merchandise with these varying rates of gross profit on cost. Lumber.......................25%...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


