If oxygen is collected over water at 20 C and 766 mm Hg, what is the partial
Question:
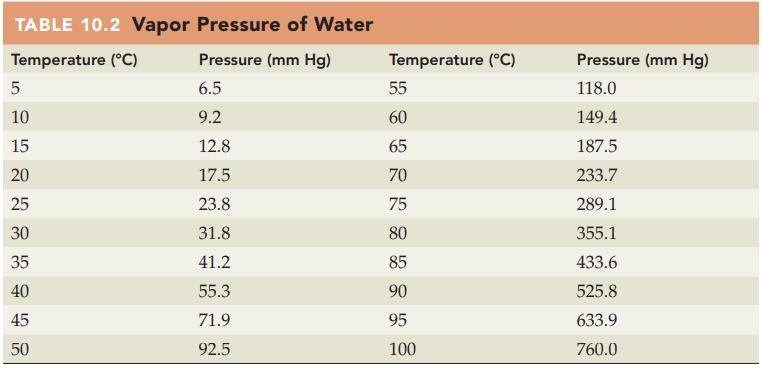
If oxygen is collected over water at 20 °C and 766 mm Hg, what is the partial pressure of the oxygen? Refer to Table 10.2 for the vapor pressure of water.
Table 10.2
Transcribed Image Text:
TABLE 10.2 Vapor Pressure of Water Temperature (°C) Pressure (mm Hg) 6.5 9.2 12.8 17.5 23.8 31.8 41.2 55.3 71.9 92.5 сл 10 15 20 25 30 35 40 45 50 Temperature (°C) DRK 85 85 55 60 65 70 75 80 90 95 100 Pressure (mm Hg) 118.0 149.4 187.5 233.7 289.1 355.1 433.6 525.8 633.9 760.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The partial pressure of oxygen in the gas mixture collected over water at 20C and 766 mm Hg is 7485 ...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
If ozone is collected over water at 30 C and 775 mm Hg, what is the partial pressure of the ozone? Refer to Table 10.2 for the vapor pressure of water. Table 10.2 TABLE 10.2 Vapor Pressure of Water...
-
If 75.0 mL of oxygen gas is collected over water at 20 C and 762 mm Hg, what is the pressure at 72.5 mL and 10 C? The vapor pressure of water at 10 C is 9.2 mm Hg.
-
Write from a perspective of a student learning econmoics, based on the songs lyrics of money- the beatles, answer the questions each in 4 sentences each What is an incentive? How does it relate to...
-
Calculate the standard entropy change for the following reactions at 25C. Comment on the sign of r S. (a) 2 Al(s) + 3 Cl 2 (g) 2 AlCl 3 (s) (b) 2 CH 3 OH() + 3 O 2 (g) 2 CO 2 (g) + 4 H 2 O(g)
-
Under Par, Inc., is an Internet retailer of golf equipment. Customers order golf equipment from the company, using an online catalog. The company processes these orders and delivers the requested...
-
The performance (in thousands of dollars) of Kenmore Airlines for the most recent year is shown in the following table. The master budget had been based on a budget of $0.20 revenue per...
-
Here is a graph using WVS data contrasting the importance of religion in the United States and Russia. Calculate the Lie Factor for the graph. Hint: Use the Russia bar as the old and the United...
-
On 1 January 2009 Henry Ltd issued a convertible debenture for 200 million carrying a coupon interest rate of 5%. The debenture is convertible at the option of the holders into 10 ordinary shares for...
-
1 Spicy Sauces, Inc., and Tom's Bottling Plant have a manufacturing franchise arrangement. This involves the transfer of Select one: O a. a trade name. O b. the formula to make a certain product. O...
-
Describe the meaning of the expression collecting a gas over water.
-
A steel cylinder with nitrogen, hydrogen, and ammonia gases is at 500 C and 5.00 atm. If the partial pressure of nitrogen is 1850 mm Hg and hydrogen is 1150 mm Hg, what is the partial pressure of...
-
Go to http://www.federalreserve.gov/aboutthefed/. Click on The Federal Reserve Board and choose Board Members. Find biographical data of members of the Board of Governors by following the link on...
-
Moving Inc. wants to develop an activity flexible budget for the activity of moving materials. Moving Inc. uses forklifts to move materials from receiving to storeroom and then to production. The...
-
We are in the tail end of Quarter 3 earnings reporting season in the U.S. markets. Roughly 60 percent of companies that have reported their Q3 earnings so far have reported negative earnings relative...
-
Below is a running shock tube illustration. 0.1 0.0 | 0.0 4 4 Diaphragm 1 0.5 Image: Shock tube Initial setup 1 3 2 1 Expansion Head Expansion Tail Slip Shock Surface Image: Running Shock Tube...
-
As you may remember, Holiday Tree Services, Inc. (HTS) has recently entered into a contract with Delish Burger (Delish), whereby HTS is to supply and decorate a Christmas tree in each of Delish...
-
Understanding various types of leadership styles is important in order to determine personal leadership styles. Reflection: Answer both Compare and contrast 2 leadership styles. State the...
-
(a) Replace the so-called two-term Taylor estimate (equation (8) in Problem 23) by the three-term result: Compute the second derivative y" in terms of f, and fy (partial derivatives of f, with...
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
What is a joint cost? What is a separable cost?
-
Distinguish between a joint product and a byproduct?
-
Why might the number of products in a joint-cost situation differ from the number of outputs? Give an example.
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


