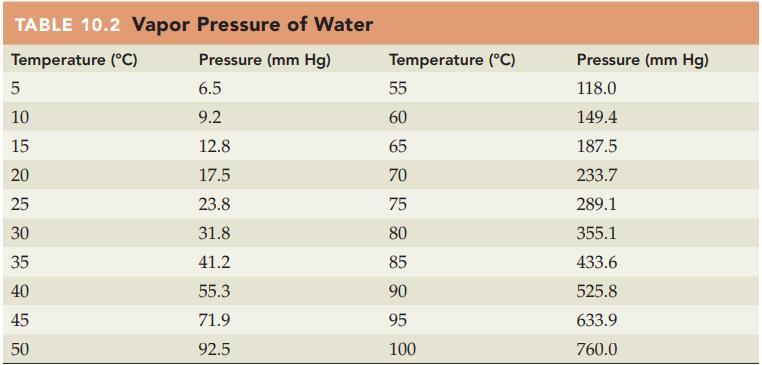
If ozone is collected over water at 30 C and 775 mm Hg, what is the partial
Question:
If ozone is collected over water at 30 °C and 775 mm Hg, what is the partial pressure of the ozone? Refer to Table 10.2 for the vapor pressure of water.
Table 10.2
Transcribed Image Text:
TABLE 10.2 Vapor Pressure of Water Temperature (°C) Pressure (mm Hg) 5 10 15 20 25 30 35 сл 40 45 50 6.5 9.2 12.8 17.5 23.8 31.8 41.2 55.3 71.9 92.5 Temperature (°C) DRK 85 85 55 60 65 70 75 80 90 95 100 Pressure (mm Hg) 118.0 149.4 187.5 233.7 289.1 355.1 433.6 525.8 633.9 760.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To find the partial pressure of ozone collected over water at 30C ...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
If oxygen is collected over water at 20 C and 766 mm Hg, what is the partial pressure of the oxygen? Refer to Table 10.2 for the vapor pressure of water. Table 10.2 TABLE 10.2 Vapor Pressure of...
-
Write from a perspective of a student learning econmoics, based on the songs lyrics of money- the beatles, answer the questions each in 4 sentences each What is an incentive? How does it relate to...
-
If 75.0 mL of oxygen gas is collected over water at 20 C and 762 mm Hg, what is the pressure at 72.5 mL and 10 C? The vapor pressure of water at 10 C is 9.2 mm Hg.
-
The slurry of Prob. 29.3 is to be filtered in a press having a total area of 8 m 2 and operated at a constant pressure drop of 2 atm. The frames are 36 mm thick. Assume that the filter medium...
-
The data related to Acclaim Sporting Goods Company's factory overhead cost for the production of 50,000 units of product are as follows: Productive capacity at 100% of normal was 75,000 hours, and...
-
A recent directive from Eugenia Yu, CEO of Comtel, has instructed each department to cut its costs by 10 percent. The traditional functional budget for the shipping and receiving department was as...
-
Using the PewSocialMedia dataset, address this question: are men more likely to use LinkedIn than women are? What happens to this relationship when you elaborate, using work status (wrkstat) as your...
-
In 2013, Adonis Industries changed its method of valuing inventory from the average cost method to the FIFO method. At December 31, 2012, Adoniss inventories were $47.6 million (average cost)....
-
Candy Jones, owner of , the Carmel Corn Shop needs your help, preparing the budget for the busy summer season. The following is what she and her management team anticipates for June, July and August...
-
What is the pressure exerted by an ideal gas at absolute zero?
-
Distinguish between a wet gas and a dry gas.
-
The global propylene industry is perfectly competitive, and each producer has the long-run marginal cost function MC(Q) = 40 12Q + Q2. The corresponding long-run average cost function is AC(Q) = 40 ...
-
Dr. Stanley and his staff are attempting to utilize effective ways to both increase the revenue for the practice and allow patients to schedule visits without a lengthy delay. Which of the following...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
If you have access to software with other methods, choose one or two of Problems and make a study (for fixed step size) of different methods. Tell which you think is best and why.
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
What is conceptually the most defensible method for allocating support-department costs? Why?
-
Distinguish between two methods of allocating common costs.
-
What role does the Cost Accounting Standards Board play when companies contract with the U.S. government?
-
The star Mira is 1.2 times the mass of the Sun and about 10,000 times more luminous than the Sun. Would Mira fit into the table above? Why or why not?
-
Which of the following was one of the most valuable benefits a company received as a sponsor of NHL games?
-
Cinder Inc. is a Canadian-controlled private corporation based in your province. The company operates a wholesale business. The following information is provided for its year ended May 31, 2023: Net...

Study smarter with the SolutionInn App


