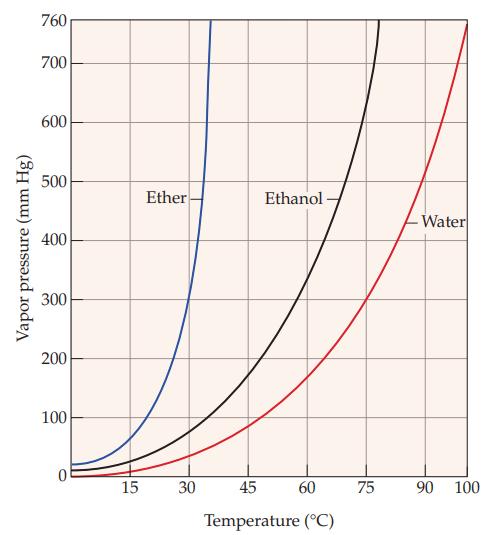
Refer to Figure 11.5 and estimate the vapor pressure of ethanol at each of the following temperatures:
Question:
Refer to Figure 11.5 and estimate the vapor pressure of ethanol at each of the following temperatures:
(a) 45 °C
(b) 60 °C
Figure 11.5
Transcribed Image Text:
Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15 Ether 30 Ethanol- 45 60 Temperature (°C) 75 -Water 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Ethanol at 45C The curve for ethanol intersects the 45C temperature line at approximately 3...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Estimate the vapor pressure of ethanol at temperatures of T = 0, 50, 100, and 150C, using the following methods. A. The Antoine equation B. The Clausius-Clapeyron equation with H vap = 42.0 kJ/mol...
-
Using the information in Problems 7.13 and 8.20, estimate the heat of vaporization for the first bit of ethanol from ethanol-water solutions containing 25, 50, and 75 mol % ethanol and from a...
-
The vapor pressure of ethanol (C2H5OH) at 20C is 44 mmHg, and the vapor pressure of methanol (CH3OH) at the same temperature is 94 mmHg. A mixture of 30.0 g of methanol and 45.0 g of ethanol is...
-
Suppose the weight of pieces of passenger luggage for domestic airline flights follows a normal distribution with u = 28 pounds and o = 5.7 pounds. (a) Calculate the probability that a piece of...
-
Is there a difference between the amount of a parents net income under the equity method and the consolidated net income for the same parent and its subsidiaries?
-
Observe the position and phase of the moon on several days in succession and at regularly chosen times during the day and evening. (It is probably best to choose a point near the first quarter of the...
-
In American roulette, the wheel has the 38 numbers 00, 0, 1, 2, , 34, 35, and 36 marked on equally spaced slots. If a player bets $1 on a number and wins, then the player keeps the dollar and...
-
In its 2009 annual report, Campbell Soup Company reports beginning-of-the-year total assets of $6,474 million, end-of-the-year total assets of $6,056 million, total sales of $7,586 million, and net...
-
A pet food manufacturer has 4,000,000 of inventory recorded at historical cost after applying lower of cost and net realizable value the company will have an inventory write down of 24,000. The...
-
What is the general relationship between the vapor pressure of a liquid and its temperature?
-
Predict which liquid in each pair has the higher surface tension: (a) CH 3 COOH or C 2 H 5 Cl (b) C 2 H 5 OH or CH 3 OCH 3 .
-
What is credit rationing, and how does it affect the choices of consumers and firms?
-
Use the Comparison Theorem to determine whether the integral is convergent or divergent. L da
-
Problem 3 (2 scenarios) Scenario 1: Rocky Inc hired a new intern from CSU to help with year-end inventory. The intern computed the inventory counts at the end of 2020 and 2021. However, the intern's...
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Solve the given system of equations graphically by using a graphing calculator. y=5x x+y2=81 Find the solution with the smaller x-value. x= y= (Type an integer or a decimal rounded to one decimal...
-
I-The market for Sony's Playstation5 game console has changed from 2021 to 2023. With restrictions from the Covid-19 pandemic ending people are finding other entertainment options available such as...
-
Three mutually exclusive alternatives are being considered: At the end of its useful life, an alternative is not replaced. If the MARR is 10%, which alternative should be selected? (a) Based on the...
-
Find the APR in each of the following cases: NUMBER OF TIMES COMPOUNDED Semiannually Monthly Weekly Infinite EAR APR 10.4% 8.9 11.6 15.4
-
In the UK initial public offerings of common stock are usually sold by an offer for sale. Mr. Bean has observed that on average these stocks are underpriced by about 9 percent and for some years has...
-
Get a hold of the prospectus for a recent IPO. How do the issue costs compare with? (a) Those of the Marvin issue and (b) Those shown in Table 15.3? Can you suggest reasons for the differences?...
-
Why are the costs of debt issues less than those of equity issues? List the possible reasons.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


