Refer to the periodic table and calculate the molar mass for each of the following. (a) Calcium
Question:
Refer to the periodic table and calculate the molar mass for each of the following.
(a) Calcium sulfide, CaS
(b) Calcium sulfate, CaSO4
(c) Dichlorine pentaoxide, Cl2O5
(d) Glycerin, C3H5 (OH)3.
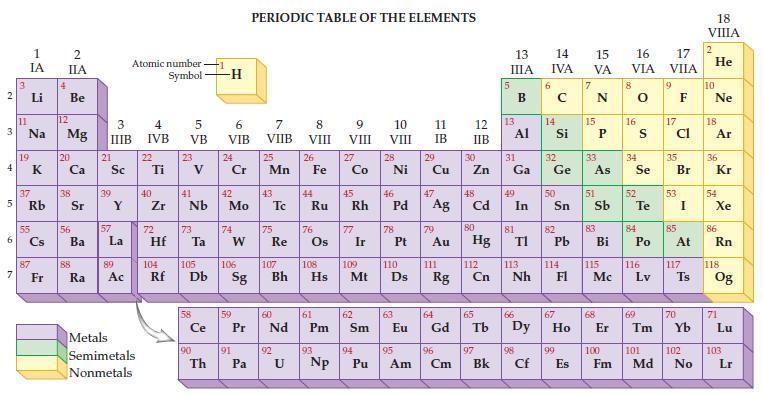
Transcribed Image Text:
2 3 4 av 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55 87 2 IIA Fr Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Atomic number Symbol Ac Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta 104 105 Rf Db Ce 90 Th -H 6 VIB 24 Cr 42 Mo 74 W 106 Sg 59 91 PERIODIC TABLE OF THE ELEMENTS Pa 7 VIIB 25 Mn 43 75 Re 60 Pr Nd 107 92 8 VIII U 26 44 108 Bh Hs Fe Ru 76 61 Pm 93 Np 9 VIII 27 Co 77 Ir 109 62 45 46 47 48 Rh Pd Ag Cd Sm 10 VIII 110 Mt Ds 94 28 Pu Ni 78 63 11 12 IB IIB 95 30 Cu Zn Am 29 79 Au 64 Eu Gd 111 Rg 96 Cm 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 31 Al Ga 49 In 81 TI 113 Nh 66 98 Cf 14 15 16 IVA VA VIA 6 14 32 Ge 50 Sn 82 33 e As Pb 114 67 99 E Es 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 cl 35 Br 53 85 At 117 Ts 70 18 VIIIA 2 He 10 Ne 18 Ar 36 54 Kr Xe 86 Rn 118 Og 71 Lu 100 101 102 103 Fm Md No Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Here are the molar masses for each compound a Calci...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
A tank contains 4lb of salt dissolved in 95 gal of water suppose that brine containing 2lb of salt per gallon of brine is allowed to enter the tank at a rate of 2t gal/min and that the mixed solution...
-
Refer to the periodic table and calculate the molar mass for each of the following. (a) Iron(II) acetate, Fe(C 2 H 3 O 2 ) 2 (b) Iron(II) phosphate, Fe 3 (PO 4 ) 2 (c) Tribromine octaoxide, Br 3 O 8...
-
Refer to the periodic table and obtain the group and period for each of the following elements. Also determine whether the element is a metal, nonmetal, or metalloid. a. S b. Fe c. Ba d. Cu e. Ne
-
Photons of wavelength 1.68 cm excite transitions from the rotational ground state to the first rotational excited state in a gas. Whats the rotational inertia of the gas molecules?
-
Nordham Corporation?s trial balance at December 31, 2012, is presented below. All 2012 transactions have been recorded except for the items described below. Unrecorded transactions 1. On January 1,...
-
Write the binomial probability in words. Then, use a continuity correction to convert the binomial probability to a normal distribution probability. P(x < 25)
-
Does the contractor have the experience needed for the type of building project you require?
-
Upper World Corporation sells tractor trailers on the installment plan. On October 1, 2014, Upper World entered into an installment-sale contract with Lower Sky Inc. for a 5-year period. Equal annual...
-
66. Unexhausted basic exemption limit of a resident individual can be adjusted against (a) only LTCG taxable @20% u/s 112 (b) only STCG taxable @15% u/s 111A (c) both (a) and (b) (d) casual income...
-
A 1-carat diamond is pure, crystalline carbon and has a mass of 0.200 g. Which has more atoms: a 1-carat diamond, or a googol (1 x 10 100 ) of atoms?
-
If 0.500 mol of yellow powder sulfur reacts with 0.500 mol of oxygen gas, what is the empirical formula of the sulfur oxide?
-
When a system is taken from stare a to stare b in Fig. 19.28 along the path acb, 90.01 of heat flows into the system and 60.0 J of work is done by the system. (a) How much heat flows into the system...
-
Suggest at least 3 touchpoints for each stage of the decision-making process that SEDO can use. Search and find in which of the touchpoints for information search stage suggested by you, can you see...
-
How do modern database management systems address the challenges posed by Big Data, including storage, processing, and analysis of massive volumes of heterogeneous data, while maintaining performance...
-
Describe how the various and sometimes seemingly unrelated topic areas work together toward managing healthcare quality
-
find Fourier series of the following functions (a) f1(x) = sinh(x), (b) f2(x) = cosh(x), (c) f3(x) = x + |x|, (d) f4(x) = x|x|.
-
Explore the realm of database transaction processing, elucidating the nuances of ACID (Atomicity, Consistency, Isolation, Durability) properties and their manifestation in ensuring transactional...
-
For the particle in a box with infinitely high walls and for the harmonic oscillator, there are no continuum eigenfunctions, whereas for the hydrogen atom we do have continuum functions. Explain this...
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
Ratio Computations and Additional Analysis Brad burn Corporation was formed 5 years ago through a public subscription of common stock. Daniel Brown, who owns 15% of the common stock, was one of the...
-
Horizontal and Vertical Analysis presented on page 1370 are comparative balance sheets for the Gilmour Company. (Round to two decimal places) (a) Prepare a comparative balance sheet of Gilmour...
-
Dividend Policy Analysis Matheny Inc. went public 3 years ago. The board of directors will be meeting shortly after the end of the year to decide on a dividend policy. In the past, growth has been...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


