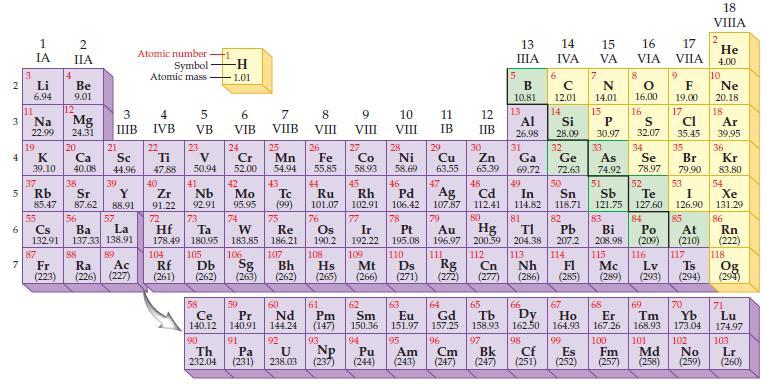
Refer to the periodic table and predict the number of valence electrons for an atom of each
Question:
Refer to the periodic table and predict the number of valence electrons for an atom of each of the following representative elements:
(a) Na
(b) Al
(c) S
(d) Xe.
Periodic Table:
Transcribed Image Text:
2 3 4 5 N.. 3 7 Li 6.94 11 1 IA Na 22.99 19 37 G 85.47 4 2 IIA Be 9.01 38 Rb Sr 87 12 K Ca Sc 39.10 40.08 44.96 87.62 56 55 6 Cs Ba Fr (223) Mg 24.31 20 21 88 39 3 IIIB Y 88.91 57 Lo 132.91 137.33 138.91 La 89 O Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 Hf Ta 178.49 180.95 104 105 ᎠᏏ Rf (261) (262) 58 Ce 140.12 90 Th 232.04 -H 1.01 6 VIB 24 Cr 52.00 42 Mo 95.95 74 W 183.85 7 VIIB 91 25 Pa (231) Mn 54.94 43 Tc (99) 75 59 60 Pr Nd 140,91 144.24 Re 186.21 8 VIII 92 U 238.03 26 Fe 55.85 44 Ru 101.07 76 Os 190.2 108 9 VIII 27 Np (237) Co 58.93 77 78 Ir 192.22 109 Mt Pt 195.08 110 Ds 106 107 Hs Rg Sg Bh Cn (263) (262) (265) (266) (271) (272) (277) 10 11 VIII IB 28 Ni 58.69 45 46 Rh Pd 102.91 106.42 61 62 63 Pm Sm Eu (147) 150.36 151.97 93 94 95 Pu Am (244) (243) 29 Cu 63.55 47 12 IIB 30 111 Zn 65.39 5 13 ΠΙΑ B 10.81 13. Al 26.98 31 Ga 69.72 48 Ag 107.87 79 Cd 112.41 80 82 Au Hg Pb 196.97 200.59 204.38 207.2 112 113 114 Nh FI (286) (285) 49 In 114.82 81 14 IVA F 7 6 C 12.01 14 15 Se 50 Si P 28.09 30.97 32 33 Ge As 72.63 74.92 78.97 51 52 Sn Sb Te 118.71 121.75 127.60 83 84 Bi Po 208.98 (209) 115 116 Mc Lv Ts (289) (293) (294) 64 66 67 65 Gd Tb Dy Ho 157.25 158.93 162.50 164.93 96 97 98 99 Cm Bk Cf (247) (247) (251) 15 VA N 14.01 16 17 VIA VIIA 68 Er 167.26 100 Es Fm (252) (257) 8 9 0 F 16.00 19.00 17 16 S 32.07 34 cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 69 70 Tm Yb 168.93 173.04 101 102 Md No (258) (259) 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Find the element in the periodic table note the group number and indicate the number of valence elec...View the full answer

Answered By

SUMAN DINDA
I LIKE TO TEACH STUDENTS. SO, I START MYSELF AS A PRIVATE TUTOR. I TEACH STUDENTS OF DIFFERENT CLASSES. I HAVE ALSO DONE BACHELOR OF EDUCATION DEGREE(B.ED). DURING THIS COURSE I HAD TO TEACH IN A SCHOOL. SO I HAVE A GOOD EXPERIENCE IN TEACHING.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The electronics industry manufactures transistors using arsenic diffusion. Refer to the periodic table and predict an element that may substitute for arsenic. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55...
-
The electronics industry manufactures semiconductor chips from silicon. Refer to the periodic table and predict an element that may substitute for silicon. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87...
-
Refer to the periodic table and predict the number of 5d electrons in a Pt atom. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 87 4 Fr (223) 2 IIA Be 9.01 12 Mg...
-
1:When developing a marketing strategy for business customers, it is essential to understand the process the business goes through when making a buying decision. Knowledge of business buying behavior...
-
Breyer Memorial Hospital received a $100,000 gift that was restricted by the donor for heart research. At fiscal year-end Breyer had incurred $25,000 in expenses related to this project. Explain how...
-
Boundary-layer analysis for fluid flow over a flat plate predicts the following relationships between the local Sherwood (Sh x ), Reynolds (Re), and Schmidt (Sc) numbers: with the transition...
-
Using globalEDGE, find the country commercial guide for two emerging markets of your choice. Compare the two countries on the following dimensions: leading sectors for exports and investment and...
-
Information concerning Montana Company is provided in BE19-16. What are the total product costs for the company under absorption costing?
-
3. Burrough Corporation concluded that the fair value of Helyar Company was $80,000 and paid that amount to acquire all of its net assets. Helyar reported assets with a book value of $60.000 and fair...
-
What is the electron dot formula for an atom of neon? (a) (b) (c) (d) (e) Ne
-
Predict the number of valence electrons for a Group VIIA/17 element. (a) 1 (b) 2 (c) 3 (d) 7 (e) 17.
-
The income elasticity of demand for private universities is closest to: A. 0.5 . B. 0.8 . C. 1.3 . The market demand function for four-year private universities is given by the equation: \[ Q_{p...
-
31. What is meant by path? 32. Give the formula for calculating D4 and D8 distance. 33. What is geometric transformation? 34. What is image translation and scaling? 35. Define the term Luminance
-
1. Explain Brightness adaptation and Discrimination 2.Explain sampling and quantization:
-
3. Explain about Mach band effect? 4. Explain color image fundamentals. 5. Explain CMY model.
-
1. Describe the fundamental steps in image processing? 2. Explain the basic Elements of digital image processing:
-
3. Explain the Structure of the Human eye 4. Explain the RGB model
-
Explain the relationship between the management functions of planning and controlling costs.
-
The cost curve for the city water supply is C(Q) = 16 + 1/4 Q2, where Q is the amount of water supplied and C(Q) is the cost of providing Q acre-feet of water. (An acre-foot is the amount of water...
-
The board of directors of Gifford Corp. declared cash dividends of $260,000 during the current year. If dividends payable was $85,000 at the beginning of the year and $90,000 at the end of the year,...
-
Explain how the amount of cash payments to suppliers is computed under the direct method.
-
The net income for Letterman Company for 2010 was $320,000. During 2010, depreciation on plant assets was $124,000, amortization of patent was $40,000, and the company incurred a loss on sale of...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


