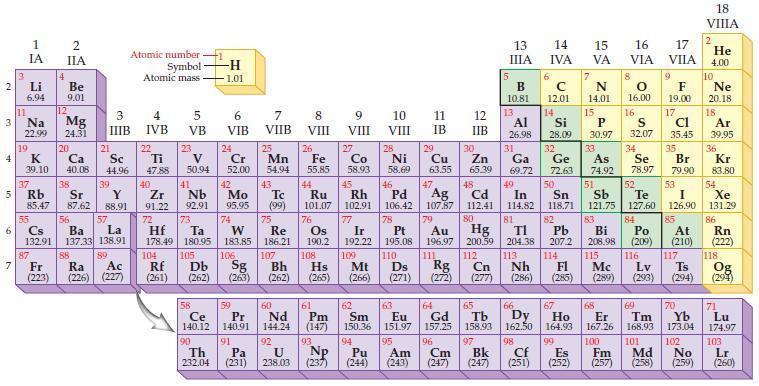
Refer to the periodic table and predict which element in each of the following pairs has the
Question:
Refer to the periodic table and predict which element in each of the following pairs has the higher ionization energy.
(a) Mg or Ca
(b) S or Se
(c) Sn or Pb
(d) N or P.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Explanation Ionization energy is the minimum amount of energy which is required t...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and predict which element in each of the following pairs has the lower ionization energy. (a) Mg or Si (b) Pb or Bi (c) Ca or Ga (d) P or Cl. Periodic Table: 2 3 4 10 6 3...
-
Refer to the periodic table and predict which element in each of the following pairs has the higher ionization energy. (a) Ga or Ge (b) Si or P (c) Br or Cl (d) As or Sb. Periodic Table: 2 3 4 10 6 3...
-
Refer to the periodic table and predict which element in each of the following pairs has the lower ionization energy. (a) Rb or Cs (b) He or Ar (c) B or Al (d) F or I. Periodic Table: 2 3 4 10 6 3 7...
-
The North American and European continents are moving apart at a rate of about 3 cm/y. At this rate how long will it take them to drift 500 km farther apart than they are at present?
-
Why do governmental fund financial statements use a different basis of accounting and measurement focus than the Governmental Activities column of the government-wide financial statements? Also,...
-
Is there anything that a Big Mac preparer in a developing country can do to earn a higher real wage rate?
-
20-1. Qu son las ventas personales ?
-
Following is information for the Fulcrum Companys three business segments located in Europe. Fulcrums applicable tax rate for the segments is 30%, and its weighted average cost of capital for each...
-
Herp Save & El Paul Swanson has an opportunity to acquire a franchise from The Yogurt Place, Incorporated, to dispense frozen yogurt products under The Yogurt Place name. Me Swanson has assembled the...
-
Which group of elements has the lowest ionization energy?
-
Draw the electron dot formula for each of the following elements. (a) He (b) Pb (c) Se (d) Ne (e) Cs (f) Ga (g) Sb (h) Br.
-
Show that x 2 + 4 is prime.
-
2. (3 points) NextGames Inc. has a new video game cassette for the upcoming holiday season. It is 3 trying to determine the target cost for the game if the selling price per unit will be set at $130,...
-
1. After watching the SR WEBINAR on how risk managers create better decision-making through a positive culture what do you think the three (or more) important points made during the webinar 2....
-
| Variance analysis, multiple products. The Robin's Basket operates a chain of Italian gelato stores. Although the Robin's Basket charges customers the same price for all flavors, production costs...
-
Question 31 of Your local coffee shop is extremely busy, so the cashier asks what you'd like to order and your name. The cashier writes this information onto a cup and passes it to the barista. After...
-
Find SSR = xy Rx+1 -dA, R= [0,2] x [4,4] Round your answer to four decimal places.
-
Donald has two investments in activities that are considered nonrental passive activities. He acquired Activity A six years ago, and it was profitable until the current year. He acquired Activity B...
-
Air pollution generated by a steel mill is an example of a) a positive production externality. b) a negative production externality. c) a public good. d) the free-rider problem. State and local taxes...
-
Describe the procedures involved in segregating various deferred tax amounts into current and noncurrent categories.
-
How is it determined whether deferred tax amounts are considered to be related to specific asset or liability amounts?
-
At the end of the year, Falabella Co. has pretax financial income of $550,000. Included in the $550,000 is $70,000 interest income on municipal bonds, $25,000 fine for dumping hazardous waste, and...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


