Refer to the periodic table and predict which of the following ions are isoelectronic with the noble
Question:
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas argon.
(a) Al3+
(b) Ca2+
(c) S2–
(d) N3–.
Periodic Table:
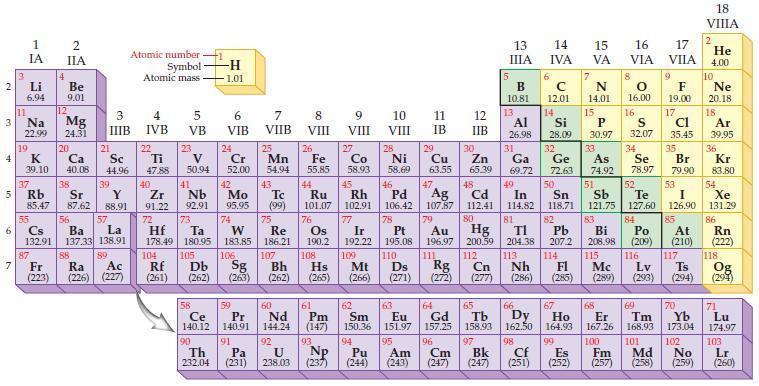
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Ts Lv (289) (293) (294) 117 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine which ions are isoelectronic with the noble gas argon Ar you need to identify ions that ...View the full answer

Answered By

Ashish Bhalla
I have 12 years work experience as Professor for Accounting, Finance and Business related subjects also working as Online Tutor from last 8 years with highly decentralized organizations. I had obtained a B.Com, M.Com, MBA (Finance & Marketing). My research interest areas are Banking Problem & Investment Management. I am highly articulate and effective communicator with excellent team-building and interpersonal skills; work well with individuals at all levels.
4.80+
17+ Reviews
46+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas xenon: (a) Cs + (b) Cl (c) La 3 + (d) Se 2 . Periodic Table: 2 3 4 15 6 7 3 11 Li 6.94 1 IA...
-
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas argon: (a) K + (b) Br (c) Ca 2+ (d) O 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4...
-
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas krypton. (a) K + (b) Sr 2+ (c) Cl (d) Se 2 .
-
We plan to remove about 90% of the A present in a gas stream by absorption in water which contains reactant B. Chemicals A and B react in the liquid as follows: B has a negligible vapor pressure,...
-
Describe the governmental activities of a state or local government and identify the measurement focus and basis of accounting used in accounting and financial reporting for these activities.
-
The Streptococcus pyogenes Cas9 endonuclease can be targeted to a specific genomic DNA sequence by an sgRNA that at its 5 end has 20 nucleotides complementary to the target sequence. If this target...
-
Unusual Values A person randomly chooses a World Series in which eight games were played and claims that this is an unusual event. Use the information in Exercise 28 to determine if this person is...
-
Sandblasting equipment acquired at a cost of $42,000 has an estimated residual value of $6,000 and an estimated useful life of 10 years. It was placed in service on October 1 of the current fiscal...
-
680 Cotton: 120 Wheat 240 Wheat 360 Wheat 480 Wheat None of the above Question 28 560 Wheat: 120 Cotton 240 Cotton 360 Cotton 480 Cotton None of the above For the next four problems, you will find...
-
Refer to the periodic table and write the predicted electron configuration for each of the following positive ions using core notation. (a) Mg 2+ (b) K + (c) Fe2 + (d) Zr 2+ . Periodic Table: 2 3 4...
-
Write the ionic charge for each of the following ions as predicted from the group number in the periodic table. (a) Cs ion (b) Ga ion (c) O ion (d) I ion. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1...
-
Pinnacle Engineering, an acclaimed chemical engineering team of engineers, chemists, and other scientists, had the following income in 2011: The following changes are expected in 2012: 1. The company...
-
What are the formulae for Static Error coefficient,Speed Error and Acceleration Error in Linear Control Systems?
-
How do we design a solenoid valve?
-
How do individual and group decision processes aid or impede business decision-making?
-
RQ2: What recent advancements have been made in the formulation and use of strategy?
-
In what circumstances does a corporation record a gain related to a distribution to a shareholder?
-
How does the organizational structure of an MNC influence its strategy implementation?
-
(EPS: Simple Capital Structure) Ott Company had 210,000 shares of common stock outstanding on December 31, 2010. During the year 2011 the company issued 8,000 shares on May 1 and retired 14,000...
-
(EPS: Simple Capital Structure) Kendall Inc. presented the following data. Net income $2,200,000 Preferred stock: 50,000 shares outstanding, $100 par, 8% cumulative, not convertible 5,000,000 Common...
-
(EPS: Simple Capital Structure) A portion of the statement of income and retained earnings of Pierson Inc. for the current year follows. Note 1 during the year, Pierson Inc. suffered a major casualty...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


