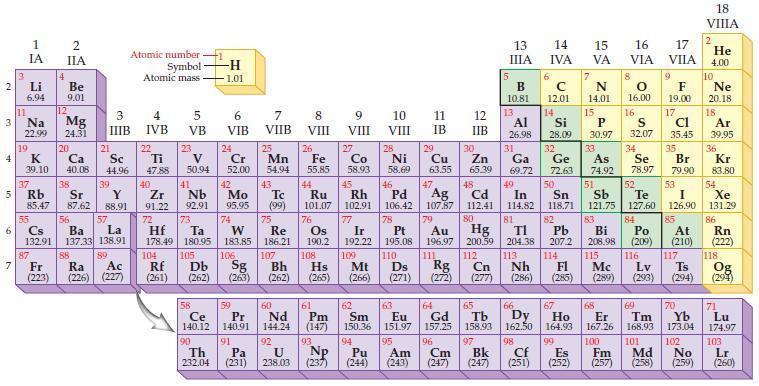
Refer to the periodic table and write the predicted electron configuration for each of the following positive
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following positive ions using core notation.
(a) Mg2+
(b) K+
(c) Fe2+
(d) Zr2+.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Mg2 Core electron configuration Ne Valence electrons 3s ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Fe 3+ (b) Se 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) Li (b) F (c) Mg (d) P (e) Ca (f) Mn (g) Ga (h) Rb. Periodic Table: 2 3 4 10 6 3 7 11...
-
Economic crises tend to occur sporadically virtually every decade and in various countries ranging from Sweden to Argentina, from Russia to Korea, and from Japan to the United States. Each crisis is...
-
Describe the fiduciary activities of a state or local government and explain how accounting and financial reporting for fiduciary activities differ from those for governmental and business-type...
-
A polypeptide consists of three separate segments of amino acids, ABC. Another polypeptide contains segments A and C but not segment B. How might you determine if these two polypeptides are produced...
-
Finding Probabilities Use the probability distribution you made for Exercise 28 to find the probability of randomly selecting a World Series that consisted of (a) four games, (b) at least five games,...
-
Weiland Co. shows the following information on its 2016 income statement: sales = $173,000; costs = $91,400; other expenses = $5,100; depreciation expense = $12,100; interest expense = $8,900: taxes...
-
Smooth Blend, Inc., a calendar year company, produces several blends of whiskey. Maturing whiskey is stored for 3 years in a large, dark aromatic warehouse owned by Smooth Blend. Smooth Blend sells...
-
Refer to the periodic table and write the predicted electron configuration for each of the following positive ions using core notation. (a) Ti 2+ (b) Zn 2+ (c) Y 3+ (d) Cs + . Periodic Table: 2 3 4...
-
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas krypton. (a) K + (b) Sr 2+ (c) Cl (d) Se 2 .
-
What are equivalent units? Why are they needed with a process costing system? (p. 160) ;
-
Plains Wars United States history
-
Oxygen has three isotopes with mass numbers of 16, 17, and 18. The atomic number of oxygen is eight. How many protons and neutrons does each of the isotopes have?
-
XYZ Inc. a calendar year, accrual basis corporation, had the following items during 2021: Gross revenue from operations Cost of goods sold $420,000 ($180,000) $9,000 LT capital gain .LT capital...
-
(Issuance, Exercise, and Termination of Stock Options) On January 1, 2010, Magilla Inc. granted stock options to officers and key employees for the purchase of 20,000 shares of the companys $10 par...
-
(Issuance, Exercise, and Termination of Stock Options) On January 1, 2009, Scooby Corporation granted 10,000 options to key executives. Each option allows the executive to purchase one share of...
-
(Accounting for Restricted Stock) Derrick Company issues 4,000 shares of restricted stock to its CFO, Dane Yapping, on January 1, 2010. The stock has a fair value of $120,000 on this date. The...
-
Justice Corporation Comparative Balance Sheet December 31, 2025 and 2024 2025 2024 Assets Current Assets: $ Cash and Cash Equivalents 2,254 $ 1,876 Justice Corporation reported the following...
-
The Fields Company has two manufacturing departments forming and painting. The company uses the FIFO method of process costing at the beginning of the month the forming department has 33.000 units in...
-
A comparative balance sheet for Lomax Company containing data for the last two years is as follows: Lomax Company Comparative Balance Sheet This Year Last Year $ 96,000 $ 70,000 640,000 672,500...

Study smarter with the SolutionInn App


