Refer to the periodic table and write the predicted electron configuration for each of the following ions
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation:
(a) Fe3+
(b) Se2–.
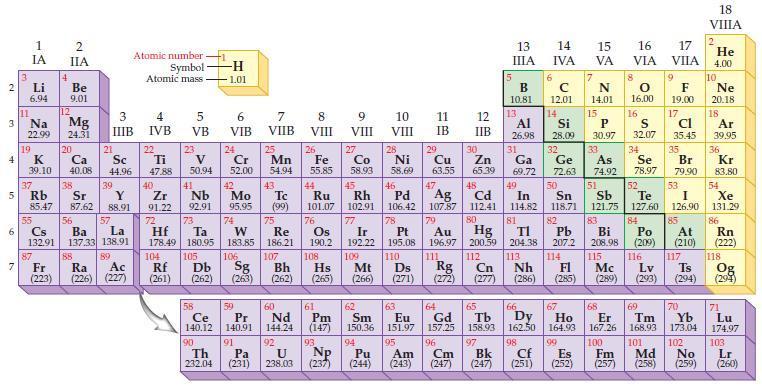
Transcribed Image Text:
2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2 IIA 87 Be 9.01 12 K Ca 39.10 40.08 Mg 24.31 20 38 21 Rb Sr Y 85.47 87.62 88.91 88 Sc 44.96 3 4 IIIB IVB 39 56 Cs La Ba 132.91 137.33 138.91 57 89 Atomic number Symbol Atomic mass Fr Ra Ac (223) (226) (227) 22 Ti 47.88 40 Zr 91.22 72 104 5 VB Rf (261) 23 V 50.94 41 Nb 92.91 Hf Ta 178.49 180.95 73 105 58 Ce 140.12 90 -H 1.01 Th 232.04 6 VIB 24 Cr 52.00 106 7 VIIB 42 Tc Mo 95.95 (99) 74 25 Db Sg Bh (262) (263) (262) 59 Pr 140.91 91 Mn 54.94 75 Re 183.85 186.21 Pa (231) 43 107 60 Nd 144.24 8 VIII 26 Fe 55.85 44 Ru 101.07 76 Os 190.2 9 VIII 92 U 238.03 (237) NP 93 45 Rh 102.91 77 10 VIII 27 28 Co Ni 58.93 58.69 63.55 65.39 Cu Zn Ir 192.22 109 62 61 Sm Pm (147) 150.36 94 Pu Am (244) (243) 46 Pd 106.42 78 63 Eu 151.97 95 11 IB 29 12 IIB 47 30 5 48 Cd 112.41 80 Hg 200.59 13 IIIA 32 33 34 Ge As Se 72.63 74.92 78.97 50 53 54 Ag 51 52 Sn Sb Te I Xe 118.71 121.75 127.60 126,90 131.29. 82 85 107.87 79 Pt Au 195.08 196.97 110 111 Ds 83 84 Pb Bi Po 207.2 208.98 (209) 108 204.38 113 Nh 115 116 112 Cn Rg 114 Fl Mc Hs Mt (265) (266) (271) (272) (277) (286) (285) (289) (293) (294) Lv B с 10.81 12.01 14 15 IVA VA N 14.01 15 13 14 Al Si P 26.98 28.09 30.97 31 6 Ga 69.72 49 In 114.82 81 7 64 66 65 67 Gd Tb Dy Ho 157.25 158.93 162.50 164.93 96 97 Cm Bk (247) (247) 16 VIA 8 0 16.00 16 S 32.07 17 VIIA 68 69 Er Tm 167.26 168.93 98 99 100 101 Cf Es Fm Md (251) (252) (257) (258) 9 F 19.00 17 Cl 35.45 35 Br 79.90 At (210) 18 VIIIA 2 117 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 86 Rn (222) 118 Ts Og (294) 70 71 Yb Lu 173.04 174.97 102 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
We refer to the periodic table to recall the blocks of elements so that we can write the electron co...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Cd 2+ (b) P 3 . Periodic Table: 2 3 4 15 6 7 3 11 Li 6.94 1 IA Na...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) Br (b) Te 2 (c) As 3 (d) O 2 . Periodic Table: 2 3 4 10...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) F (b) S 2 (c) N 3 (d) I . Periodic Table: 2 3 4 10 6 3...
-
The stress field (15.1.7) for the screw dislocation produces no tangential or normal forces on a cylinder of finite radius with axis along the dislocation line (z-axis). However, show that if the...
-
Fund balance with U.S. Treasury. One amount is missing in the following trial balance of proprietary accounts, and another is missing from the trial balance of budgetary accounts of a certain agency...
-
Consider the circuit shown in Fig. Q26.12. What happens to the brightnesses of the bulbs when the switch S is closed if the battery (a) Has no internal resistance and (b) Has nonnegligible internal...
-
Use at least two different methodologies to develop as accurate a forecast for the demand as possible. Use each of those methods to project the next four months demand. LO.1
-
Incomplete manufacturing costs, expenses, and selling data for two different cases are as follows. Instructions (a) Indicate the missing amount for each letter. (b) Prepare a condensed cost of goods...
-
Homework help for these Accounting questions of ABC Company. Please show all your work, will upvote! Round all interim calculations to 4 decimal places. 16 ) a) ABC gross payroll for April is $65000....
-
Predict the next ion in the isoelectronic series: Se 2 , Br , Kr, Rb + , Sr 2+ .
-
What is the term for the elements in the series that follows element 57?
-
Refer to Samsungs balance sheet in Appendix A. How does its cash (titled Cash and cash equivalents) compare with its other current assets (both in amount and percent) as of December 31, 2013? Compare...
-
Question 4 25 p J Mart is considering purchasing a new inventory control system featuring state-of-the-art technology. Two vendors have submitted proposals to supply J Mart with the new system. The...
-
ME 2352 Design Optimization Assignment TWO, due February 6th, 2024, 4:00 pm University of New Brunswick Department of Mechanical Engineering 1. By use of definition of linear dependency determine if:...
-
IKEA's People and Planet Positive sustainability plan, launched in 2012, aims to contribute to a better life for people and a better future for the planet. The plan outlines several sustainable goals...
-
Question 4 [25 marks] A cantilever beam AB is fixed to a wall and is subjected to concentrated and distributed loads as shown in figure B1. a) Draw the free-body diagram of the problem. [5 marks] a)...
-
GMC is an Australian farm machinery manufacturer, operating since 1975. The company makes high-quality farm machinery and equipment including a range of slashers, mowers, aerators, mulchers and...
-
Under which circumstances is a fixed salary preferable to a pure bonus compensation system?
-
The Adjusted Trial Balance columns of a 10-column work sheet for Webber Co. follow. Complete the work sheet by extending the account balances into the appropriate financial statement columns and by...
-
Identify the lease classifications for lessors and the criteria that must be met for each classification.
-
Outline the accounting procedures involved in applying the direct-financing method.
-
Outline the accounting procedures involved in applying the operating method by a lessor.
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


