Refer to the periodic table and write the predicted electron configuration for each of the following negative
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation.
(a) Br–
(b) Te2–
(c) As3–
(d) O2–.
Periodic Table:
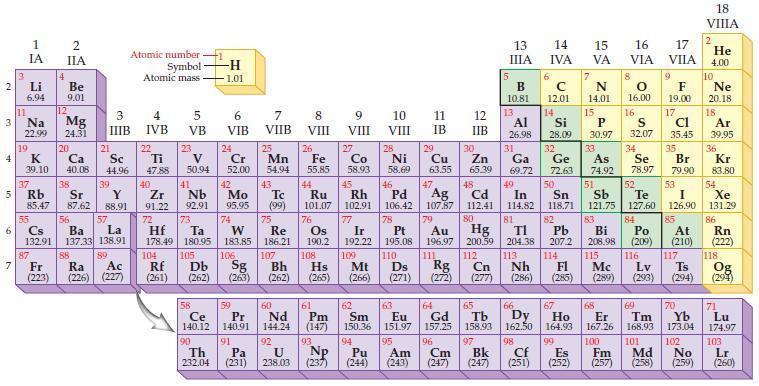
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Here are the predicted electron configurations for the given negative ions a Br Bromi...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Fe 3+ (b) Se 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) Li (b) F (c) Mg (d) P (e) Ca (f) Mn (g) Ga (h) Rb. Periodic Table: 2 3 4 10 6 3 7 11...
-
Discuss the two-pipe system, how it works, and its advantages and disadvantages.
-
What are the three categories of funds prescribed by GASB standards and which fund types are included in each? Do the three fund categories correspond precisely with the three activity categories...
-
The Ti plasmid contains a region referred to as T-DNA. Why is this region called T-DNA, and what is its significance?
-
Household Size The histogram shows the distribution of household sizes in the United States for a recent year. (Adapted from U.S. Census Bureau)
-
Refer to the data for Provost Industries in Case 419. Assume that the company uses the FIFO method in its process costing system. Required: 1. Prepare a report for the Finishing Department for April...
-
Question 32\ Miriam Company made an error in booking journal entries this month. The receipt of cash for services was recorded as a debit to Cash for $42 and a credit to Service Revenue for $425 ....
-
Use the American convention to designate the group number corresponding to each of the following groups listed by the European convention. (a) Group IA (b) Group IB (c) Group IIIA (d) Group IIIB.
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) F (b) S 2 (c) N 3 (d) I . Periodic Table: 2 3 4 10 6 3...
-
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them: a. b. c. d. e. f. g. h. i. cis-2-butene and trans-2-butene j. CH3CH2CH2OH...
-
1. State the difference between lists and tuples in Python programming. 2. Explain why Python is an Interpreted Language
-
1. How does Python handle memory? 2. Python's ternary operators: how do they work? 3. How is Python's multithreading implemented
-
In a relational database, explain the difference between Inner join & Outer join. Provide an example query for each and describe the result set produced by each query.
-
Expand the following condensed structures to show the covalent bonds and lone-pair electrons: a. CH3NHCH2CH3 b. (CH3)2CHCl c. (CH3)2CHCHO d. (CH3)3C(CH2)3CH(CH3)2
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
(Conversion of Bonds) Schuss Inc. issued $3,000,000 of 10%, 10-year convertible bonds on June 1, 2010, at 98 plus accrued interest. The bonds were dated April 1, 2010, with interest payable April 1...
-
(Conversion of Bonds) Gabel Company has bonds payable outstanding in the amount of $400,000, and the Premium on Bonds Payable account has a balance of $6,000. Each $1,000 bond is convertible into 20...
-
(Conversion of Bonds) On January 1, 2010, when its $30 par value common stock was selling for $80 per share, Bartz Corp. issued $10,000,000 of 8% convertible debentures due in 20 years. The...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


