Refer to the periodic table and write the predicted electron configuration for each of the following negative
Question:
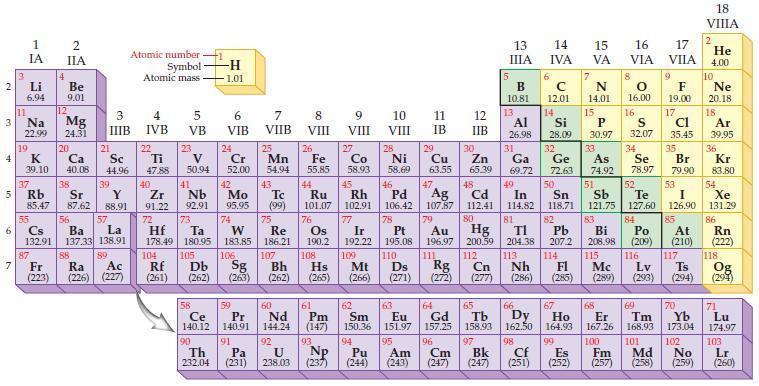
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation.
(a) F–
(b) S2–
(c) N3–
(d) I–.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Here is the explanation for each ion a F Fluorine has 9 electrons When it ...View the full answer

Answered By

SATYAPRAKASH YADAV
I HAD DONE MY B.TECH IN CIVIL ENGINEERING IN 2018 FROM DR.A P J ABDUL KALAM TECHNICAL UNIVERSITY . MY TEACHING EXPERIENCE IS APPROXIMATELY 3 YEARS .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Fe 3+ (b) Se 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) Li (b) F (c) Mg (d) P (e) Ca (f) Mn (g) Ga (h) Rb. Periodic Table: 2 3 4 10 6 3 7 11...
-
If an investor holds a credit-linked note and the credit event does not occur, the investor receives: A. All promised cash flows as scheduled. B. All coupon payments as scheduled but not the par...
-
If a discrete presentation is used for the financial data of a component unit in the statement of net assets of a governmental financial reporting entity, there is no need for the component unit to...
-
Why do null mutations in the msl gene in Drosophila have no effect in females?
-
Carpooling The histogram shows the distribution of carpooling by the number of cars per household. (Adapted from Federal Highway Administration)
-
A negotiable promissory note executed and delivered by B to C passed in due course and was indorsed in blank by C, D, E, and F. G, the present holder, strikes out Ds indorsement. What is the...
-
Important task, no plagiarism please! Develop a business plan according to requirements below: Business Description: Introduction to business- define what the business is, startup capital, business...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) Br (b) Te 2 (c) As 3 (d) O 2 . Periodic Table: 2 3 4 10...
-
Refer to the periodic table and write the predicted electron configuration for each of the following positive ions using core notation. (a) Ti 2+ (b) Zn 2+ (c) Y 3+ (d) Cs + . Periodic Table: 2 3 4...
-
All future costs are relevant. Do you agree? Explain briefly.
-
What Is Chemical Energy? Definition and Examples
-
The fundamental concern of computer science is determining what can and cannot be automated. The earliest foundations of what would become computer science predate the invention of the modern digital...
-
History of the United States
-
United States History Pearl Harbor attack
-
a. Draw two Lewis structures for C2H6O. b. Draw three Lewis structures for C3H8O.
-
D Which of the following is considered part of the Controlling activity of managerial accounting? O Choosing to purchase raw materials from one supplier versus another O Choosing the allocation base...
-
(Conversion of Bonds) The December 31, 2010, balance sheet of Osygus Corp. is as follows. 10% callable, convertible bonds payable (semiannual interest dates April 30 and October 31; convertible into...
-
(Conversion of Bonds) On January 1, 2009, Trillini Corporation issued $3,000,000 of 10-year, 8% convertible debentures at 102. Interest is to be paid semiannually on June 30 and December 31. Each...
-
(Issuance of Bonds with Warrants) Prior Inc. has decided to raise additional capital by issuing $175,000 face value of bonds with a coupon rate of 10%. In discussions with investment bankers, it was...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


