Refer to the periodic table and write the predicted electron configuration for each of the following elements.
Question:
Refer to the periodic table and write the predicted electron configuration for each of the following elements.
(a) Li
(b) F
(c) Mg
(d) P
(e) Ca
(f) Mn
(g) Ga
(h) Rb.
Periodic Table:
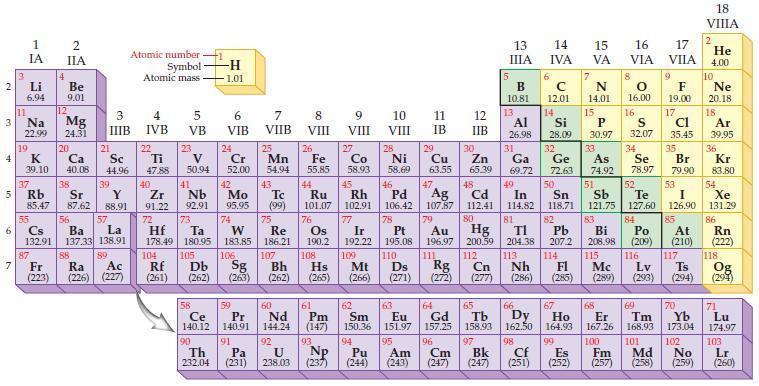
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Lithium Li Atomic Number 3 Predicted Electron Configuration 1s 2s b Fluorine F Atomic Number ...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and write the predicted electron configuration for each of the following elements. (a) B (b) Ti (c) Na (d) O (e) Ge (f) Ba (g) Pd (h) Kr. Periodic Table: 2 3 4 10 6 3 7 11...
-
Refer to the periodic table and write the predicted electron configuration for each of the following ions using core notation: (a) Fe 3+ (b) Se 2 . 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2...
-
Refer to a periodic table and write the predicted electron configuration for each of the following elements by counting the number of electrons in each block: (a) P (b) Co. Periodic Table: 2 3 4 5 6...
-
Scenic Ventures is considering an investment in a start-up firm offering ecotourism excursions in Costa Rica. Scenic Ventures estimates that, if the venture is successful, the ecotourism company will...
-
Bartling Energy Systems recently reported $9,250 of sales, $5,750 of operating costs other than depreciation, and $700 of depreciation. The company had no amortization charges, it had $3,200 of...
-
A horizontal rope with 15 N tension drags a 25 kg box 2.0 m to the left across a horizontal surface. How much work is done by (a) Tension (b) Gravity?
-
2 En los primeros 50 aos de operaciones comerciales, Johnson Carpet Company produjo alfombras para uso residencial. La fuerza de ventas estaba estructurada en forma geogrfica. Durante los ltimos...
-
How might XYZ Software Company arrive at the values in the above table? For each entry, describe the process of determining the cost per incident and frequency of occurrence.
-
Use the Black-Scholes formula to calculate the price of a call option given the following information: S = $1.50 / , X = $1.55 / , r = 1% , r = 2% , T = 1 , = 20%
-
Which energy sublevel is being filled by the lanthanide series?
-
What type of energy sublevel (s, p, d, f) is being filled by the inner transition elements?
-
What is Title VII? What does it state?
-
Lazlo s estimates uncollectible accounts to be 0 . 9 % of sales. Its year - end unadjusted trial balance shows Accounts Receivable of $ 1 1 2 , 5 0 0 and sales of $ 9 6 5 , 0 0 0 . If Lazlo s uses...
-
Identify one or two of the best and one or two of the worst work teams on which you served as a member. 1. Identify the top three to five factors that made the team the best or the worst in terms of...
-
ColorCoder is a HousePaint Shop which supplies currently two types of house paints, namely, alpha and beta house paints. The shop is planning to sell a primer (paint base) and the needed paint...
-
which department adds value to a product or service that is observable by a customer?
-
Using Figure 14.1, answer the following questions: a. What was the settle price for July 2022 coffee futures on this date? What is the total dollar value of this contract at the close of trading for...
-
How often must employers report payroll taxes to the IRS? What form must the employer file?
-
On January 2, 20X3, Sheldon Bass, a professional engineer, moved from Calgary to Edmonton to commence employment with Acco Ltd., a large public corporation. Because of his new employment contract,...
-
(One Temporary Difference, Future Taxable Amounts, One Rate, Beginning Deferred Taxes) Brennan Corporation began 2010 with a $90,000 balance in the Deferred Tax Liability account. At the end of 2010,...
-
(Three Differences, Compute Taxable Income, Entry for Taxes) Havaci Company reports pretax financial income of $80,000 for 2010. The following items cause taxable income to be different than pretax...
-
(Two Temporary Differences, One Rate, Beginning Deferred Taxes) The following facts relate to Alschuler Corporation. 1. Deferred tax liability, January 1, 2010, $40,000. 2. Deferred tax asset,...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


