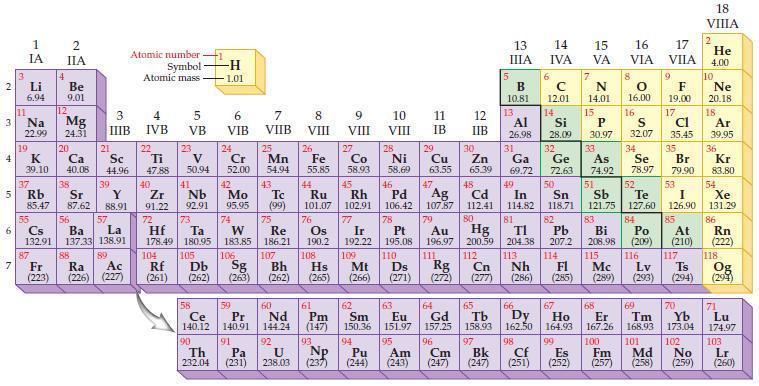
Refer to the periodic table and select the symbol of the element that fits each of the
Question:
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions.
(a) The semimetal in the second period
(b) The semimetal in the fourth period and Group IVA/14
(c) The halogen that exists as yellow gas
(d) The halogen that is radioactive.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Element Symbols based on Descriptions a Boron B This is the only semimetal in the second pe...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The semimetal in the third period (b) The semimetal in the fourth period and Group...
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The fourthperiod alkali metal (b) The fourthperiod alkaline earth metal (c) The rare...
-
Refer to the periodic table and select the symbol of the element that fits each of the following descriptions. (a) The thirdperiod alkali metal (b) The thirdperiod alkaline earth metal (c) The rare...
-
Determine if the following strains satisfy the compatibility equations (2.6.2): a. b. c. where A, B and C are constants. Equation 2.6.2 ex=Ay, ey = ez = 0, exy = (Ax+Bz)/2, eyz = Bxz + Cy, ezx = C.x
-
Single Audit. Background, Mountain Lake Mental Health Affiliates, a nongovernmental not-for-profit organization, has contacted Rill Wise, CPA, about conducting an annual audit for its first year of...
-
A beaker of water at room temperature is placed in an enclosure, and the air pressure in the enclosure is slowly reduced. When the air pressure is reduced sufficiently, the water begins to boil. The...
-
If the old forecast is 100 and the latest actual demand is 85, what is the exponentially smoothed forecast for the next period? Alpha is 0.2. LO.1
-
When will the world run out of oil? One way to judge is to determine the oil reserves of the countries around the world. The next table displays the known oil reserves of the top 15 countries....
-
5. A couple needs $32,000 for a car. To save up to buy the car for the aforementioned price, they decide to invest 2,000 at the end of every quarter into a bank account that accrues 10% interest...
-
Identify the group number corresponding to each of the following families of elements. (a) Boron group (b) Oxygen group (c) Nickel group (d) Copper group.
-
Identify the group number corresponding to each of the following families of elements. (a) Alkali metals (b) Alkaline earth metals (c) Halogens (d) Noble gases.
-
Simplify the given algebraic expressions. 3{2.1e 1.3[f 2(e 5 f )]}
-
1- What is the chairman of the Texas State Board of Education's primary occupation? 2- Why are the decisions the Texas State Board of Education make about their high school science standards so...
-
SECTION 7 KEEPING ENTIRE STRUCTURES IN EQUILIBRIUM ASSIGNMENT #2 1. Find the reactions at A and C. A 2. Find the reactions at A and C. 60.0 kN B 3.00 9.00 4.00 6.00 5.00 kN 3.00 B 1.00 2.00 10.0 kN...
-
The year-end financial statements of Python Tax Services are provided below. Service revenue Expenses: Salaries Utilities Insurance Supplies Net income PYTHORT TAX SERVICES Income Statement $ 68,500...
-
a. Calculate the volume of the solid of revolution created by rotating the curve y=2+4 exp(-5 x) about the x-axis, for x between 2 and 4. Volume: b. The equation of a circle of radius r, centered at...
-
The simply supported timber beam of Figure 1 is made-up by gluing together three 300 mm by 150 mm planks as shown. The beam has to carry a uniformly distributed vertically downward load of 8 kN/m for...
-
Identify some conditions where upper management might allow some divisions to have a lower required rate of return.
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
(Preparation of Pension Worksheet) Using the information in E20-2 prepare a pension worksheet inserting January 1, 2010, balances, showing December 31, 2010, balances, and the journal entry recording...
-
(Basic Pension Worksheet) The following facts apply to the pension plan of Boudreau Inc. for the year 2010. Using the preceding data, compute pension expense for the year 2010, as part of your...
-
(Application of Years-of-Service Method) Andrews Company has five employees participating in its defined-benefit pension plan. Expected years of future service for these employees at the beginning of...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


