Refer to the periodic table and state the atomic mass (in amu) of one atom for each
Question:
Refer to the periodic table and state the atomic mass (in amu) of one atom for each of the following metals.
(a) Sodium
(b) Strontium
(c) Silicon
(d) Selenium.
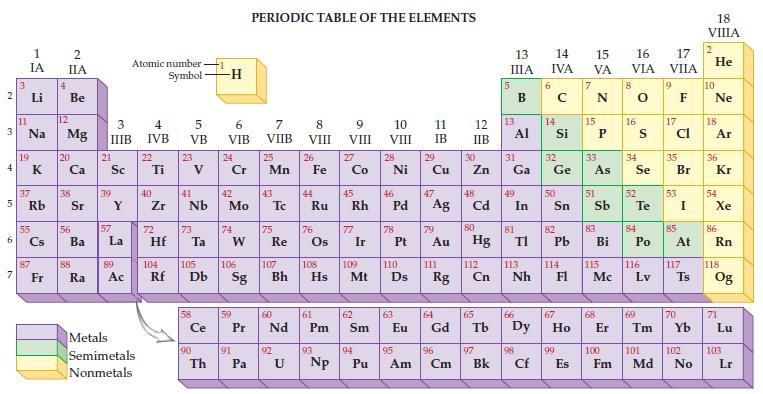
Transcribed Image Text:
2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -H Th 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 60 Pr Nd Re 92 8 VIII U 26 44 Fe Ru 76 61 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Pm 93 9 VIII Np 27 Co 77 Ir 62 Sm 10 VIII 45 46 47 Rh Pd Ag 94 28 Pu Ni 78 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 13 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 13 IIIA Bk 31 Al Ga 49 In 81 TI 113 Nh 66 98 Cf 14 15 16 IVA VA VIA 6 14 50 82 Pb 32 33 34 Ge As Se 114 67 99 E 15 Es 51 83 Bi 115 Mc 68 8 16 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 cl 35 Br 53 85 At 117 Ts 70 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Based on the periodic table a Sodium Na 2298977 amu b Strontium ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the atomic mass (in amu) of one atom for each of the following nonmetals. (a) Beryllium (b) Barium (c) Boron (d) Bromine. 2 3 4 AD 6 3 7 11 1 IA 37 5 Rb Li Na 19...
-
Refer to the periodic table and state the mass for each of the following number of atoms. (a) 1 atom of carbon (b) 6.02 x 10 23 atoms of carbon. 2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2...
-
Element 43 is used in medical radiology to locate tumors. Refer to the periodic table and state whether Tc has any stable isotopes. Periodic Table: 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2...
-
You find a certain stock that had returns of 18 percent, 23 percent, 16 percent, and 9 percent for four of the last five years. If the average return of the stock over this period was 10.3 percent,...
-
On January 1, 2012, the ledger of Montoya Company contains the following liability accounts. Accounts Payable....... $52,000 Sales Taxes Payable...... 7,700 Unearned Service Revenue... 16,000 During...
-
Assume the same facts as in Problem C:13-48 and that before Yuji's death in 2018 his wife already owned property valued at $300,000. Assume that each asset owned by each spouse increased 8% in value...
-
In what way is the accounting for a foreign currency borrowing more complicated than the account ing for a foreign currency account payable? LO9
-
A company manufactures various sized plastic bottles for its medicinal product. The manufacturing cost for small bottles is $52 per unit (1,000 bottles), including fixed costs of $15 per unit. A...
-
[7]. Which of the following is NOT true? (Assume that present values are calculated from the end of the life of the option to today.) Answer: _______ A. An American call option is always worth less...
-
What is the mass of 7.75 x 10 22 formula units of lead(II) sulfide, PbS?
-
State the number of atoms for each of the following metals. (a) 63.55 g Cu (b) 40.08 g Ca
-
A 6-kg object is pulled along a frictionless horizontal surface by a horizontal force of 10 N. (a) If the object is at rest at t = 0, how fast is it moving after 3 s? (b) How far does it travel...
-
As of June 30, 2012, the bank statement showed an ending balance of \(\$ 13,879.85\). The unadjusted Cash account balance was \(\$ 13,483.75\). The following information is available: 1. Deposit in...
-
An engineering study has developed the following cost data for the production of product A: (1) If the current production level is 1,500 units, what is the incremental cost of producing an additional...
-
On December 1, a group of individuals formed a corporation to establish the Local, a neighborhood weekly newspaper featuring want ads of individuals and advertising of local firms. The free paper...
-
Design an arithmetic circuit with one selection variable S and two n-bit data inputs A and B. The circuit generates the following four arithmetic operations in conjunction with the input carry C in ....
-
For the system you chose for Problems and Exercises 3, complete section 4.0, A-C, Management Issues, of the BPP Report. Why might people sometimes feel that these additional steps in the project plan...
-
A one-dimensional double-well potential has V = for x < -1/2 l, V = 0 for -1/2 l x -1/4 l, V = V0 for -1/4 l < x < 1/4 l, V = 0 for 1/4 l x 1/2 l, and V = for x > 12 l, where l and V0 are...
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Discuss how a change to the LIFO method of inventory valuation is handled when it is impracticable to determine previous LIFO inventory amounts.
-
How should consolidated financial statements be reported this year when statements of individual companies were presented last year?
-
Simms Corp. controlled four domestic subsidiaries and one foreign subsidiary. Prior to the current year, Simms Corp. had excluded the foreign subsidiary from consolidation. During the current year,...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


