Refer to the periodic table and state the mass for each of the following number of atoms.
Question:
Refer to the periodic table and state the mass for each of the following number of atoms.
(a) 1 atom of carbon
(b) 6.02 x 1023 atoms of carbon.
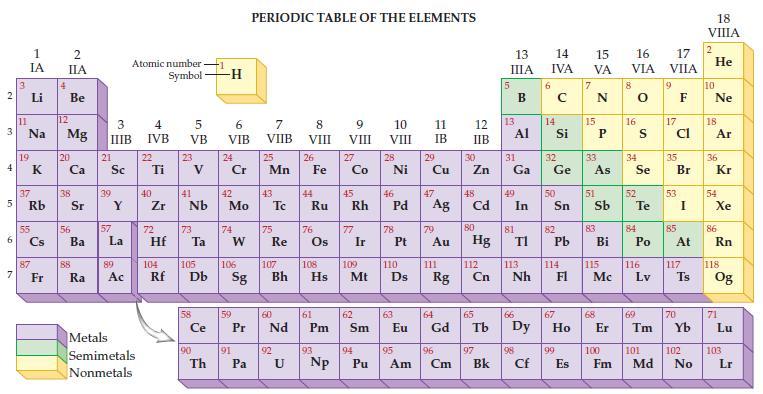
Transcribed Image Text:
2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -H Th 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 60 Pr Nd Re 92 8 VIII U 26 44 Fe Ru 76 61 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Pm 93 9 VIII Np 27 Co 77 Ir 62 Sm 10 VIII 45 46 47 Rh Pd Ag 94 28 Pu Ni 78 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 13 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 13 IIIA Bk 31 Al Ga 49 In 81 TI 113 Nh 66 98 Cf 14 15 16 IVA VA VIA 6 14 50 82 Pb 32 33 34 Ge As Se 114 67 99 E 15 Es 51 83 Bi 115 Mc 68 8 16 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 cl 35 Br 53 85 At 117 Ts 70 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a A carbon atom has ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the mass of 6.02 x 10 23 atoms of each of the following metals. (a) Sodium (b) Strontium (c) Silicon (d) Selenium. 2 3 4 av 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55...
-
Refer to the periodic table and state the mass of 6.02 x 10 23 atoms of each of the following nonmetals. (a) Beryllium (b) Barium (c) Boron (d) Bromine. 2 3 4 AD 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55...
-
Refer to the periodic table and state the number of atoms for each of the following nonmetals. (a) 30.97 g P (b) 20.18 g Ne. Periodic Table: 19 2 3 4 5 6 7 3 11 1 IA Li 6.94 Na 22.99 19 37 4 12 87 2...
-
One end of a light, elastic string, of natural length 1.2m and modulus of elasticity 32N, is attached to a fixed point, B. A particle, P, of mass 1.5 kg, is then attached to the other end of the...
-
Alvarez Company has the following data for the weekly payroll ending January 31. Employees are paid 1 times the regular hourly rate for all hours worked in excess of 40 hours per week. FICA taxes are...
-
On September 15, 2008, Lehman Brothers filed for Chapter 11the largest such bankruptcy in American history. The move stunned global financial markets and, many argue, triggered the ensuing financial...
-
On July 1, 2009, Houghton Company borrowed 200,000 euros from a foreign lender evidenced by an interest-bearing note due on July 1, 2010. The note is denominated in euros. The U.S. dollar equivalent...
-
The fiscal year for Hokkaido Company ends on May 31. Results for the year ended May 31, 20X1, included the following (in millions of Japanese yen except for number of shares outstanding): Cash and...
-
Mark's Consulting experienced the following transactions for Year 1 , its first year of operations, and Year 2 . Assume that all transactions involve the receipt or payment of cash. Transactions for...
-
How many atoms of silver equal a mass of 107.87 amu? (a) 1 (b) 47 (c) 107.87 (d) 108 (e) 6.02 x 10 23 .
-
Chlorine is prepared industrially by heating hydrogen chloride gas with oxygen gas. Assuming the only products are water and chlorine gas, write a balanced chemical equation for the manufacture of...
-
Fraser Baker opened Baker?s Accounting Service in Winnipeg on September 1, 2021. On September 30, the balance sheet showed Cash $5,700; Accounts Receivable $2,100; Supplies $350; Equipment $7,600;...
-
Repeat Prob. 10-18 for signed-magnitude binary numbers. Prob. 10-18 Derive an algorithm in flowchart form for the comparison of two signed binary numbers when negative numbers are in signed-2's...
-
Tideview Home Health Care, Inc., has a bond issue outstanding with eight years remaining to maturity, a coupon rate of 10 percent with interest paid annually, and a par value of $1,000. The current...
-
Captain Billy Whirlywhirl Hamburgers issued 7%, 10-year bonds payable at 70 on December 31, 2010. At December 31, 2012, Captain Billy reported the bonds payable as follows: Captain Billy Whirlywhirl...
-
Two scenarios about the future of the global economy in 2050 have emerged. Known as continued globalization, the first scenario is a (relatively) rosy one. Spearheaded by Goldman Sachs, whose...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Nike: Associating Athletes, Performance, and the Brand highlights Nike's use of marketing research...
-
Use the Numerov method to find the lowest three energy eigenvalues for a one-particle system with V = cx4, where c is a constant. Use either a program similar to that in Table 4.1, a spreadsheet, or...
-
Data 9.2 on page 540 introduces the dataset Cereal, which includes information on the number of grams of fiber in a serving for 30 different breakfast cereals. The cereals come from three different...
-
Refer to the accounting change by Wertz Construction Company in BE22-1. Wertz has a profit-sharing plan, which pays all employees a bonus at year-end based on 1% of pre-tax income. Compute the...
-
Shannon, Inc., changed from the LIFO cost flow assumption to the FIFO cost flow assumption in 2010. The increase in the prior years income before taxes is $1,200,000. The tax rate is 40%. Prepare...
-
Tedesco Company changed depreciation methods in 2010 from double-declining-balance to straight-line. depreciation prior to 2010 under double-declining-balance was $90,000, whereas straight line...
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...

Study smarter with the SolutionInn App


