Refer to the periodic table and state the mass of 6.02 x 10 23 atoms of each
Question:
Refer to the periodic table and state the mass of 6.02 x 1023 atoms of each of the following nonmetals.
(a) Beryllium
(b) Barium
(c) Boron
(d) Bromine.
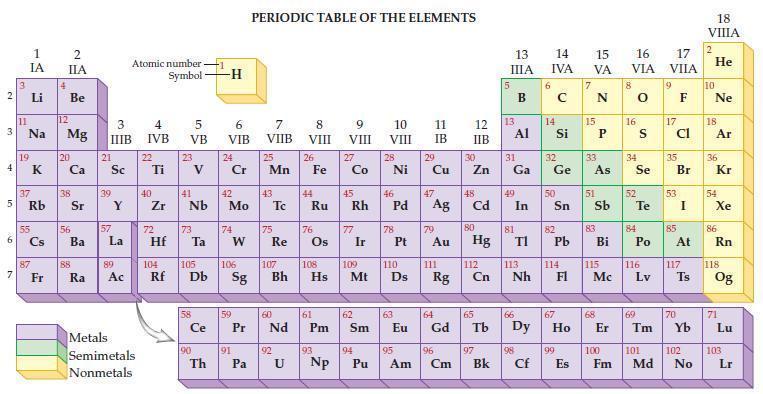
Transcribed Image Text:
2 3 4 AD 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55 87 2 IIA Fr Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Atomic number Symbol Ac Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta 104 105 Rf Db 90 -H Th 6 VIB 24 Cr 42 Mo 74 W 106 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 59 60 Ce Pr Nd 75 Re 92 8 VIII U 26 107 108 Sg Bh Hs 44 Fe Ru 76 61 Pm 93 Np 9 VIII 27 Co 45 Rh 77 Ir 109 62 Sm 94 10 VIII 110 Mt Ds Pu 28 Ni 78 63 46 47 48 Pd Ag Cd 11 IB 95 Am 29 30 Cu Zn 79 Au 64 Eu Gd 111 Rg 12 IIB 96 Cm 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 Al 31 2 Ga 49 81 In TI 113 66 Nh 98 Cf 14 15 16 IVA VA VIA 6 14 32 Ge 50 82 Pb 114 33 e As 67 99 E Es 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 35 Br 53 85 At 117 Ts 70 18 VIIIA 2 He 10 Ne 18 Ar 36 54 Kr Xe 86 Rn 118 Og 71 Lu 100 101 102 103 Fm Md No Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To find the mass of 602 x 1023 atoms of each element well need their respective ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the mass of 6.02 x 10 23 atoms of each of the following metals. (a) Sodium (b) Strontium (c) Silicon (d) Selenium. 2 3 4 av 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55...
-
Refer to the periodic table and state the mass for each of the following number of atoms. (a) 1 atom of carbon (b) 6.02 x 10 23 atoms of carbon. 2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2...
-
What would you suggest to be done and which principles are important to you in this decision? How would you think about the greatest good in this case? As a leader what are your duties and who are...
-
Describe the three ways a client can reference a name from a namespace in C++.
-
The balance sheet for Focus Consulting reports the following information on July 1,2012. Focus decides to redeem these bonds at 101 after paying semiannual interest. Prepare the journal entry to...
-
What percent of U.S. adults ages 25 and over who are not high school graduates are unemployed? Use the contingency table, and the information below. Relative frequencies can also be calculated based...
-
What is foreign exchange risk? How does a company become exposed to foreign exchange risk? LO9
-
If a projectile is fired due east from a point on the surface of Earth at a northern latitude λ with a velocity of magnitude V0 and at an angle of inclination to the horizontal of a, show that...
-
On September 30 Partner C buys of partner As interest in the equal AB calendar-year partnership, so A and C each own 25% after the sale. For the year, the partnership earns income of $2,000 a month...
-
What is the number of molecules in 1.00 mol of a diatomic nonmetal?
-
Calculate the number of moles of potassium in 1.25 x 10 21 atoms K. Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit...
-
Practice in connection with unaudited historical cost financial statements is conducted by which firms? a. International accounting firms only. b. Regional- and local-size public accounting firms. c....
-
Beginning with Eq. (11.16), prove that Data from Eq. 11.16 Data from Eq. 11.21 where we have defined D8 = - 3 2 F = FiFi T = F + F + F Y = F8. 3 Show that this leads to Eq. (11.21) with the...
-
Consider the light bulb that is the object in Figure 33.28. If you move the bulb to the left, does the image shift left, shift right, or stay in the same place? Data from Figure 33.28 (a) The three...
-
Two models of light emitted from a light bulb are illustrated in Figure P33.5. (a) Describe the difference in the behavior of light in each model. (b) Describe an experiment that can determine which...
-
Parallel red and green laser rays are incident on a glass slab as shown in Figure P33.24. Sketch the rays as they pass through the slab and after they have entered the air to the right of the slab....
-
Consider the following five operations: constructing a luxury cruise ship, operating a casual dining restaurant, staging a professional sports match, manufacturing a patented drug, and rescuing...
-
For the ground state of the one-dimensional harmonic oscillator, find the average value of the kinetic energy and of the potential energy; verify that (T) = (V) in this case.
-
Privitera and Freeman (2012) constructed a scale to measure or estimate the daily fat intake of participants; the scale was called the estimated daily intake scale for fat (EDIS-F). To validate the...
-
Navajo Corporation traded a used truck (cost $20,000, accumulated depreciation $18,000) for a small computer worth $3,300. Navajo also paid $500 in the transaction. Prepare the journal entry to...
-
Use the information for Navajo Corporation from BE10-8. Prepare the journal entry to record the exchange, assuming the exchange lacks commercial substance.
-
Mehta Company traded a used welding machine (cost $9,000, accumulated depreciation $3,000) for office equipment with an estimated fair value of $5,000. Mehta also paid $3,000 cash in the transaction....
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


