Calculate the number of moles of potassium in 1.25 x 10 21 atoms K. Strategy Plan STEP
Question:
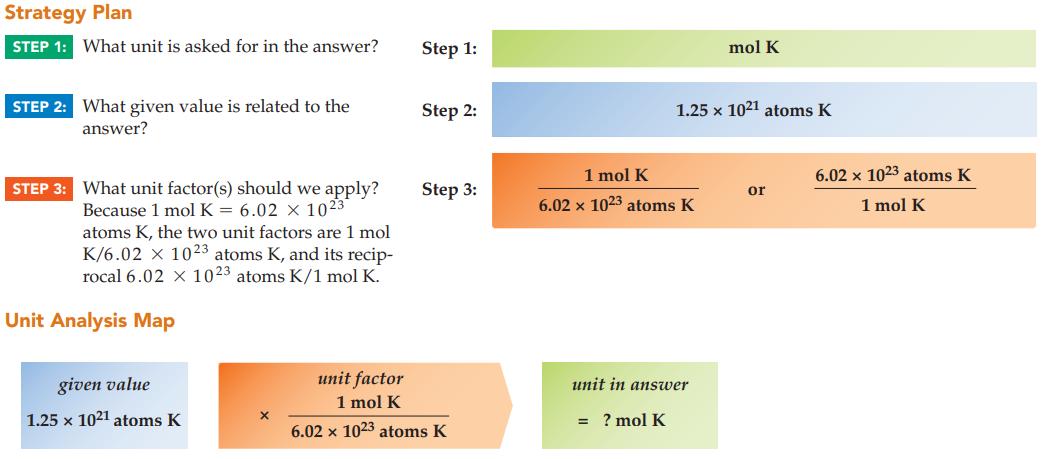
Calculate the number of moles of potassium in 1.25 x 1021 atoms K.
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? Because 1 mol K = 6.02 x 1023 atoms K, the two unit factors are 1 mol K/6.02 x 1023 atoms K, and its recip- rocal 6.02 x 1023 atoms K/1 mol K. Unit Analysis Map given value 1.25 x 1021 atoms K Step 1: Step 2: Step 3: unit factor 1 mol K 6.02 x 1023 atoms K 1 mol K 6.02 x 1023 atoms K 1.25 x 1021 atoms K unit in answer = ? mol K mol K or 6.02 x 1023 atoms K 1 mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The answer is rounded to three digits because the given v...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
In a neutralisation experiment, 25 cm3 of dilute sulfuric acid was required to react completely with 40 cm 3 of a solution of 0.25 mol dm 3 potassium hydroxide. a. Write a balanced chemical equation...
-
Castle Entertainment sells tv/movie memorabilia. The owner (who still wishes to remain anonymous but goes by the name of Richard) has decided that now is right time to once again update his...
-
A friend asks if you would be willing to check several homework problems to see if she is on the right track. Following are the problems and her proposed solutions. When you identify the problem with...
-
At the beginning of the year, Anna began a calendar-year business and placed in service the following assets during the year: Asset Date Acquired Cost Basis Computers 1/30 $28,000 Office desks 2/15...
-
Beetown Company has issued three different bonds during 2012. Interest is payable semiannually on each of these bonds. 1. On January 1, 2012, 1,000, 8%, 5-year, $1,000 bonds dated January 1, 2012,...
-
The contingency table shows the results of a random sample of public elementary and secondary school teachers by gender and years of full-time teaching experience. At = 0.01, can you conclude that...
-
How do foreign currency derivatives such as forward contracts and options hedge foreign exchange risk? LO9
-
Ricardos Mexican Restaurant incurred salaries expense of $ 65,000 for 2014. The payroll expense includes employer FICA tax, in addition to state unemployment tax and federal unemployment tax. Of the...
-
Dr. Jones maintains her books on a cash basis. At year end, her CPA converts them to an accrual basis. Dr. Jones provides the following information regarding her cash basis income for 2019: Cash...
-
Refer to the periodic table and state the mass of 6.02 x 10 23 atoms of each of the following nonmetals. (a) Beryllium (b) Barium (c) Boron (d) Bromine. 2 3 4 AD 6 3 7 11 1 IA 37 5 Rb 4 Li B Na 19 55...
-
What is the mass of 3.30 x 1023 atoms of silver, Ag? (a) 0.549 g (b) 25.8 g (c) 59.1 g (d) 85.7 g (e) 197 g.
-
A Carnot heat engine with a thermal efficiency of 60% receives heat from a source at a rate of 3000 kJ/min, and rejects the waste heat to a medium at 300 K. Determine (a) The power that is generated...
-
If Technical Specification 2 were reduced in the next design for this product, what would likely happen to customer opinion of Value Feature A? Quick Start QFD Matrix 2 Strong positive correlation...
-
Customer opinion of Value Feature B is most strongly correlated with what technical specification? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation == Strong negative...
-
Consider Quick Start QFD Matrix 1 above. Of the two value features, which do cus- tomers consider three times more important? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some...
-
Which technical spec can be most easily modified without changing current choices for the other two technical specs? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some positive...
-
Use Table A.1 to select 20 three-digit random numbers. Did any of the numbers occur more than once? How is it possible for a number to occur more than once? Make a stem-and-leaf plot of the numbers...
-
(a) If f(x) is an even function that is everywhere differentiable, prove that f -(x) is an odd function. Do not assume that f(x) can be expanded in a Taylor series. (b) Prove that the derivative of...
-
What is beacon marketing? What are digital wallets?
-
Cheng Company traded a used truck for a new truck. The used truck cost $30,000 and has accumulated depreciation of $27,000. The new truck is worth $37,000. Cheng also made cash payment of $36,000....
-
Slaton Corporation traded a used truck for a new truck. The used truck cost $20,000 and has accumulated depreciation of $17,000. The new truck is worth $35,000. Slaton also made cash payment of...
-
Ottawa Corporation owns machinery that cost $20,000 when purchased on July 1, 2007. Depreciation has been recorded at a rate of $2,400 per year, resulting in a balance in accumulated Depreciation of...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


