Refer to the periodic table inside the front cover of this textbook. State the mass of Avogadros
Question:
Refer to the periodic table inside the front cover of this textbook. State the mass of Avogadro’s number of atoms for each of the following metals.
(a) Silver
(b) Mercury
Periodic Table:
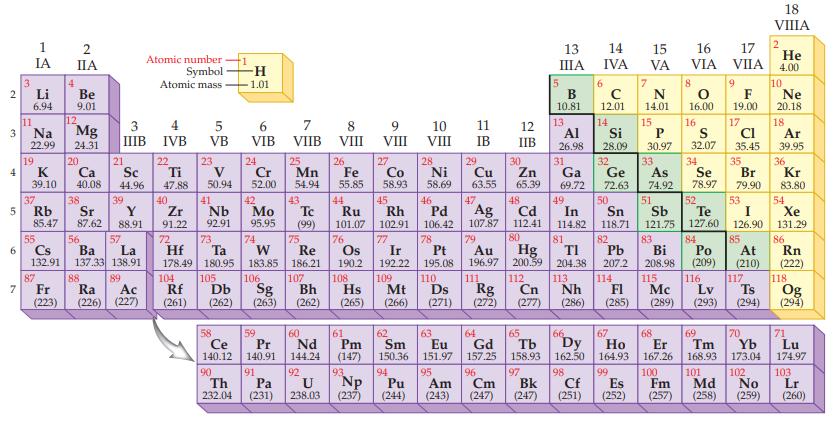
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li 6.94 Na 22.99 19 37 4 2 IIA 87 12 Fr (223) Be 9.01 Mg 24.31 20 K Ca Sc 39.10 40.08 44.96 38 21 Rb Sr Y 85.47 87.62 88.91 3 4 IIIB IVB 55 56 57 Cs Ba La 132.91 137.33 138.91 88 39 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 22 Ti 47.88 40 Zr 91.22 5 VB 23 50.94 41 Nb 92.91 -H -1.01 104 105 ᎠᏏ Rf (261) (262) 6 VIB 24 Cr 52.00 42 Mo 95.95 74 106 Sg (263) 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 59 58 Pr Ce 140.12 90 Th 232.04 (231) 140.91 91 Pa 7 VIIB 25 Mn 54.94 43 Tc (99) 75 60 Nd 144.24 92 8 VIII U 238.03 26 Fe 55.85 44 77 107 Ir 192.22 109 Bh Hs Mt (262) (265) (266) 108 76 Os 190.2 Ru Rh 101.07 102.91 61 Pm (147) 9 VIII 93 27 Np (237) Co 58.93 45 10 VIII 28 Ni 58.69 63 62 Sm Eu 150.36 151.97 94 95 Am Pu (244) (243) 11 IB 29 Cu 63.55 47 13 IIIA 64 65 Gd Tb 157,25 158.93 96 97 Cm Bk (247) (247) 5 16 15 12 Al Si IIB 26.98 28.09 32 30 31 46 52 53 Ag P S 30.97 32.07 33 34 Zn Ga Ge As Se Br 65.39 69.72 72.63 74.92 78.97 79.90 48 49 50 51 Cd In Sn Sb Te I 107.87 112.41 114.82 118.71 121.75 127.60 126.90 80 81 82 Au Pb 196.97 200.59 204.38 207.2 110. 111 112 113 114 Ds Rg Cn Nh FI (271) (272) (277) (286) (285) Pd 106.42 78 79 Pt 195.08 Hg ΤΙ B 10.81 13 14 IVA 6 с 12.01 14 7 15 VA N 14.01 16 17 He VIA VIIA 4.00 8 18 VIIIA 9 10 F Ne 16.00 19.00 20.18 2 17 18 Cl Ar 35.45 39.95 35 36 Kr 83.80 54 Xe 131.29 86 83 84 85 Bi Po At 208.98 (209) (210) 116 117 115 Mc Lv Ts (289) (293) (294) Rn (222) 118 Og (294) 66 70 71 174.97 67 68 69 Dy Ho Er Tm Yb Lu 162.50 164.93 167.26 168.93 173.04 98 99 100 101 102 Cf Es Fm Md (251) (252) (257) (258) No (259) 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
The atomic mass of each element is listed below its symbol in the periodic table The mass of Avogadr...View the full answer

Answered By

Robert Mbae
I have been a professional custom essay writer for the last three years. Over that period of time, I have come to learn the value of focusing on the needs of the clients above everything else. With this knowledge, I have worked hard to become an acclaimed writer that can be trusted by the customers to handle the most important custom essays. I have the necessary educational background to handle projects up to the Ph.D. level. Among the types of projects that I've done, I can handle everything within Dissertations, Project Proposals, Research Papers, Term Papers, Essays, Annotated Bibliographies, and Literature Reviews, among others.
Concerning academic integrity, I assure you that you will receive my full and undivided attention through to the completion of every essay writing task. Additionally, I am able and willing to produce 100% custom writings with a guarantee of 0% plagiarism. With my substantial experience, I am conversant with all citation styles ranging from APA, MLA, Harvard, Chicago-Turabian, and their corresponding formatting. With all this in mind, I take it as my obligation to read and understand your instructions, which reflect on the quality of work that I deliver. In my paper writing services, I give value to every single essay order. Besides, whenever I agree to do your order, it means that I have read and reread your instructions and ensured that I have understood and interpreted them accordingly.
Communication is an essential part of a healthy working relationship. Therefore, I ensure that I provide the client with drafts way long before the deadline so that the customer can review the paper and comment. Upon completion of the paper writing service, the client has the time and right to review it and request any adjustments before releasing the payment.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Lucia Company has set the following standard cost per unit for direct materials and direct labor. Direct materials (15 pounds @ $5 per pound) Direct labor (3 hours @ $15 per hour) During May the...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VIA element is a metalloid? b. What is the Group III A element in Period 3? Figure...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VA element is a metal? b. What is the Group IIA element in Period 3? Figure 2.15...
-
A health care facility in a metropolitan area is interested in the efficiency of its laboratory turnaround time. Based on data collected over last year, the mean turn around time was found to be 55...
-
Kroger Co.??s 2009 financial statements contained the following data (in millions). InstructionsCompute these values:(a) Working capital. (b) Currentratio. Current assets Total assets Current...
-
Download the rejected loans dataset of LendingClub data titled RejectStatsA Ready from the Connect website and do an Excel PivotTable by state; then figure out the number of rejected applications for...
-
Lawrence Company ordered parts costing FC100,000 from a foreign supplier on May 12 when the spot rate was $0.20 per FC. A one-month forward contract was signed on that date to purchase FC 100,000 at...
-
The following activities take place at Lohman CPAs during a recurring external audit of Reliance Corp. Classify the activities as value-added or non-value-added from the perspective of Reliance Corp....
-
Question Graph the annual interest rate (%) for each year. Question Graph the annual interest rate (%) for each year
-
Balance each of the following combustion reactions by inspection. (a) CH 4 O(l) + O 2 (g) CO 2 (g) + H 2 O(g) (b) C 3 H 8 O(l) + O 2 (g) CO 2 (g) + H 2 O(g).
-
Refer to the periodic table and state the mass of Avogadros number of atoms for each of the following nonmetals. (a) Sulfur (b) Helium. Periodic Table: 19 2 3 4 5 6 7 3 11 1 IA Li 6.94 Na 22.99 19 37...
-
Explain environmental constraints that conflict with the primary goal of multinational companies.
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
The Morse function U = De[1 - e-a(R-Re)]2 is often used to approximate the U(R) curve of a diatomic molecule, where the molecule's equilibrium dissociation energy De is De K U() - U(Re). (a) Verify...
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
Change in PrincipleInventory Methods Whitman Company began operations on January 1, 2008, and uses the average cost method of pricing inventory. Management is contemplating a change in inventory...
-
Accounting Change Ramirez Co. decides at the beginning of 2010 to adopt the FIFO method of inventory valuation. Ramirez had used the LIFO method for financial reporting since its inception on January...
-
Accounting Change Linden Company started operations on January 1, 2006, and has used the FIFO method of inventory valuation since its inception. In 2012, it decides to switch to the average cost...
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


