Refer to the values in Figure 12.9 and calculate the electronegativity difference in a SI bond. Figure
Question:
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a S—I bond.
Figure 12.9
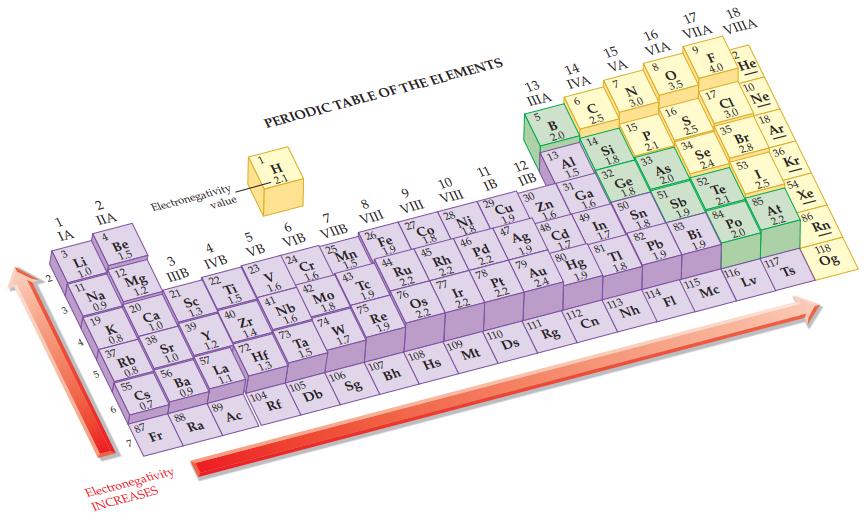
Transcribed Image Text:
1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3 Electronegativity 0.7 87 IIIB 21 Sr 1.0 56 Fr Sc Electronegativity INCREASES 39 Ba 0.9 88 1,3 value 4 IVB 22 Y 1.2 57 Ra Ti 1.5 40 La 1.1 89 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1.6 41 Hf 13 104 6 VIB 24 Nb 1.6 73 Rf Cr 1.6 42 Ta 15 7 VIIB 105 25 Mo 1.8 74 Db Mp 1.5 43 W 1.7 8 VIII 106 26 Tc 1.9 75 Sg Fe 19 44 Re 1.9 107 9 VIII 27 Ru 2.2 76 Bh 18 45 Os 2.2 10 VIII 108 28 Rh 22 77 Hs N 46 Ir 22 109 11 IB 29 Pd Mt 22 78 Cu 19 47 Pt 22 110 12 IIB 13 ΠΙΑ 5 130 Ag CE FOR 1.9 Ds 79 B 2.0 Zn 1.6 13 48 Au 111 24 14 IVA 6 AI 1.5 31 Cd 17 Rg 80 с 2.5 14 Ga 1.6 49 112 Hg 15 VA Cn Si 18 In 17 7 32 81 N 3.0 Ge 1.8 F 15 50 113 1.8 16 VIA 8 P 21 Nh 33 Sn 18 82 0 3.5 16 As 20 51 114 Pb 19 17 VIIA FI S 2.5 Sb 1.9 9 34 83 Se 24 Bi 19 F 12 4.0 He 17 52 115 18. VIIIA CL Mc 3.0 35 Te 2.1 84 10 Br 2.8 53 Po 20 116 Ne 18 I 25 85 Lv 36 Kr At 2.2 117 (54 Xe श Ts 86 Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The electronegativity values for sulfur S and iodine I on the Pauling scale ...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a HP bond. Figure 12.9 1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a SbCl bond. Figure 12.9 1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in a BCl bond. Figure 12.9 1 IA S Li 1.0 11 2 IIA 4 Na 0,9 19 Be 1.5 12 0.8 37 Mg 1.2 6 20 Rb 0.8 55 Ca 1.0 38 3...
-
11. Which one is not a source for collecting references? Ans : O Proquest Ebscohost O Scopus Safe assign
-
1. If numerous funds are maintained, which of the following transactions would typically not be reported in a municipalitys general fund? a. The collection of property taxes b. The purchase of office...
-
A companys most recent free cash flow to equity was $100 and is expected to grow at 5% thereafter. The companys cost of equity is 10%. Its WACC is 8.72%. What is its current intrinsic value?
-
Random Number Table Use the twelfth row of Table 1 in Appendix B to generate 10 random numbers between 1 and920.
-
The April 2010 income statement for Fabio's Fashions has just been received by Diana Caffrey, Vice-President of Marketing. The firm uses a variable costing system for internal reporting purposes. The...
-
what do I have wrong h. Determine the depreciation on the canoes purchased on December 2 using straight-line depreciation. Assume the useful life of the canoes is 4 years and the residual value is $0
-
Label the polar GeCl bond using delta notation ( + and ).
-
Explain why the radius of a chloride ion (181 pm) is about twice that of a chlorine atom (99 pm).
-
Finding Missing Data A new computer virus (AcctBGone) destroyed most of the company records at BackupsRntUs. The computer experts at the company could recover only a few fragments of the companys...
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Which projects should be done if the budget is $100,000? What is the opportunity cost of capital? Life Annual Salvage Project years) First Cos Value 20 20 30 15 25 0 15 $20,000 $4000 20,000 3200...
-
Is it a breach of fiduciary duty for a director of a real estate investment trust (REIT) negotiating a joint venture on behalf of the REIT with another director for the development of a portfolio of...
-
IPO Pricing the Eyetech IPO was underpriced by about 54 percent. Should Eyetech be upset at Merrill Lynch over the under-pricing?
-
IPO Pricing in the previous question, would it affect your thinking to know that the company was incorporated less than four years earlier, had only $30 million in revenues for the first nine months...
-
In the previous two questions, how would id affect your thinking to know that in addition to the 6.5 million shares offered in the IPO, Eyetech had an additional 32 million shares outstanding? Of...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


