The chloride in an aqueous sample of BaCl 2 is precipitated with 50.0 mL of 0.100 M
Question:
The chloride in an aqueous sample of BaCl2 is precipitated with 50.0 mL of 0.100 M AgNO3. The excess silver nitrate is titrated with 17.0 mL of 0.125 M K2CrO4. Calculate the mass of the barium chloride in the sample.
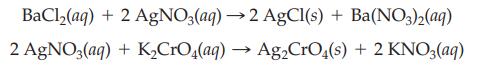
Transcribed Image Text:
BaCl₂(aq) 2 AgNO3(aq) + 2 AgNO3(aq) →2 AgCl(s) + Ba(NO3)2(aq) + K₂CrO4(aq) → Ag₂CrO4(s) + 2 KNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
the balanced equation ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Silver nitrate reacts with strontium chloride in an aqueous precipitation reaction. What are the formulas of silver nitrate and strontium chloride? Write the molecular equation and net ionic equation...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Aoslia is a small country that takes the world price of corn as given. Its domestic supply and demand for corn are given by the following: a. Assume initially that Aoslia does not open to trade. What...
-
Reza Inc. operates a retail operation that purchases and sells snowmobiles, amongst other outdoor products. The company purchases all merchandise inventory on credit and uses a periodic inventory...
-
Write a function print() that prints a vector of ints to cout. Give it two arguments: a string for labeling the output and a vector.
-
Which of the following is not a reason that firms need to innovate? a. Changing customer needs b. Preventing declining sales c. Diversifying risk d. Responding to short product life cycles e. All of...
-
Authors Academic Publishing faces three potential contingency situations, described below. Authors fiscal year ends December 31, 2015. Required: Determine the appropriate means of reporting each...
-
21 es Activity Cash paid to purchase property Cash from selling merchandise Cash paid for income taxes Cash received from a long-term note Cash paid to repurchase stock Cash received from sale of...
-
The president of a college reports to a board of trustees, and three vice-presidents report to the college president. The chief financial officer reports directly to the board of trustees. Draw a...
-
What is the molar chloride ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 25.0 mL of 0.100 M potassium chloride?
-
Let each matrix in act on C 2 . Find the eigenvalues and a basis for each eigenspace in C 2 . 0 -8 4
-
The following table contains the monthly operating costs of a company. Salary is not included. Determine the variance and standard deviation of the costs. Enero Febrero Marzo Abril Mayo Junio Julio...
-
Becker & Smith, CPAs, performs a financial statement review for BAM Markets ( BAM ) . Caroline, the manager on the job, learns that Don, a member of the review team, violated the independence rules....
-
Presented here are selected transactions for Sheridan Inc. during August of the current year. Sheridan uses a perpetual inventory system. It estimates a return rate of 10%, based on past experience....
-
. Complete both parts (a) and (b) below. ). In1 (a) Let X11, X12, ..., X be a random sample of size n from a population with mean and variance . Let X21, X22,..., X2n2 be a random sample of size n...
-
41. Let S be the cone z = x + y, z 2, oriented with outward unit normal. Use Stokes' theorem to evaluate the flux integral for the vector field SJ (V x F). ndS F(x, y, z) = (x y)i + 2zj + xk. -
-
Find the mean, median, and mode for each data set. a. Time for pizza delivery (min): {28, 31, 26, 35, 26} b. Yearly rainfall (cm): {11.5, 17.4, 20.3, 18.5, 17.4, 19.0} c. Cost of a small popcorn at...
-
Describe a job you have had in the past or a job you are very familiar with. Indicate the negative aspects of the job and how it could be improved with current human resource management techniques.
-
Why should the timing of direct materials purchases be closely coordinated with the production budget?
-
a. Discuss the purpose of the cash budget. b. If the cash for the first quarter of the fiscal year indicates excess cash at the end of each of the first two months, how might the excess cash be used?
-
How does a schedule of collections from sales assist in preparing the cash budget?
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


