Use the periodic table to predict an ionic charge for each of the following metal ions. (a)
Question:
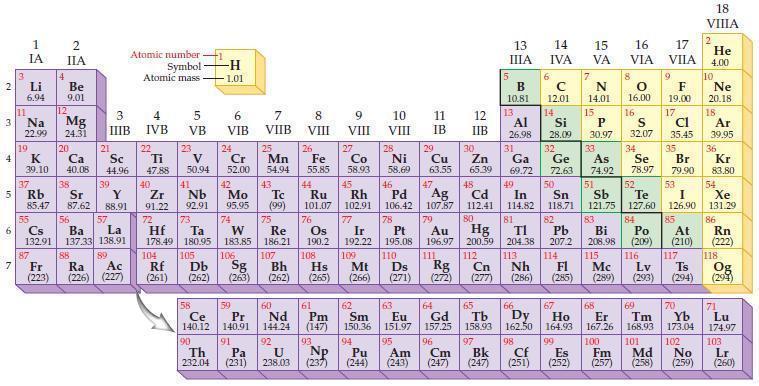
Use the periodic table to predict an ionic charge for each of the following metal ions.
(a) Li ion
(b) Mg ion
(c) Al ion
(d) Sn ion.
Periodic Table
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts Po (209) 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
In general the ionic charge of a metal ion can be predicted by looking at the metals group number ve...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Use the periodic table to predict an ionic charge for each of the following metal ions. (a) Na ion (b) Ba ion (c) Ga ion (d) Pb ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55...
-
Use the periodic table to predict the ionic charge for each of the following nonmetal ions. (a) Cl ion (b) I ion (c) Se ion (d) P ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al...
-
Use the periodic table to predict the ionic charge for each of the following nonmetal ions. (a) F ion (b) Br ion (c) S ion (d) N ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55...
-
EBIT $ 600,000 g 0.0% r 10.0% Shares o/s 200,000 Taxes 25.0% NI $ 450,000 Before Recapitalization: Value of Ops $ 4,500,000 Value per share $ 22.50 EPS $ 2.25 1:13 sthn 1h dialexh Recap: New debt...
-
Jason, Kelly, and Becky, who share partnership profits 50 percent, 30 percent, and 20 percent, respectively, decide to liquidate their partnership. They need the cash from the partnership as soon as...
-
Genesis Ltd was incorporated three years ago and has grown rapidly since then. The rapid rate of growth has created problems for the business, which the directors have found difficult to deal with....
-
Why should quality management include both the products and processes of a project? AppendixLO1
-
Use the methods of descriptive statistics to learn about the customers who visit the Heavenly Chocolates website. Include the following in your report. 1. Graphical and numerical summaries for the...
-
Question 1 (7 marks) (Note this question is from the Week 5 Tutorial) Discuss the five (5) principal functions of a modern and efficient stock exchange, which most developed or developing countries...
-
From the trends in the periodic table, apply delta notation and label each atom in the following polar covalent bonds: (a) NO (b) BrF.
-
State whether the representative particle in each of the following substances is an atom, a formula unit, or a molecule. (a) Acetone, C 3 H 6 O (b) Cobalt, Co (c) Magnetite, Fe 3 O 4 (d) Phosphorus,...
-
What are the differences in the main research objectives of exploratory, descriptive, and causal research designs? Which design type would be most appropriate to address the following question: How...
-
Over the past 40 years, union membership has declined, and it continues to do so. Instead, many companies are turning to alternative dispute resolution. We know one of the best union avoidance...
-
Article "A Leader's Journey" by Pamela Kruger Photographs by Nigel Dickson. For this discussion, let's try and unpack the key factors that led to his transformation. 1. What are your key takeaways...
-
Describe the collaborative roles of the team leader and the team coach in helping a group of people come together to form a team. Recommend strategies for Alex as team leader to use in helping to...
-
a. Complete the table with all marginal totals and cell counts. b. Calculate the following probabilities. i. For a male to be a Republican. ii. For a voter to be female. iii. For a voter to be either...
-
1. Will the Coronavirus Pandemic Make Working from Home the New Normal?" Address the following below. Define the problem described in this case. What are the management, organization, and technology...
-
The Ogi Corporation, a construction company, purchased a pickup truck for $14,000 and used MACRS depreciation in the income tax return. During the time the company had the truck, they estimated that...
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Job costing, accounting for manufacturing overhead, budgeted rates. The Lynn Company uses a job-costing system at its Minneapolis plant. The plant has a Machining Department and an Assembly...
-
Job costing, consulting firm. Taylor & Associates, a consulting firm, has the following condensed budget for 2009: Taylor has a single direct-cost category (professional labor) and a single...
-
Service industry, time period used to compute indirect cost rates. Printers, Inc. produces annual reports and marketing materials for large companies. There are three categories of costs in its...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...
-
Pedro lives in Puerto Rico and had a net taxable income of $35,000 for the year 20X1. Your gross income totals $60,000. What is Pedro's regular income tax for 20X1? a.$4,620 b.$4,900 c.$2,318 d.$2,520
-
The change in cash is equal to the change in liabilities less the change in equity plus the change in noncash assets. O True False

Study smarter with the SolutionInn App


