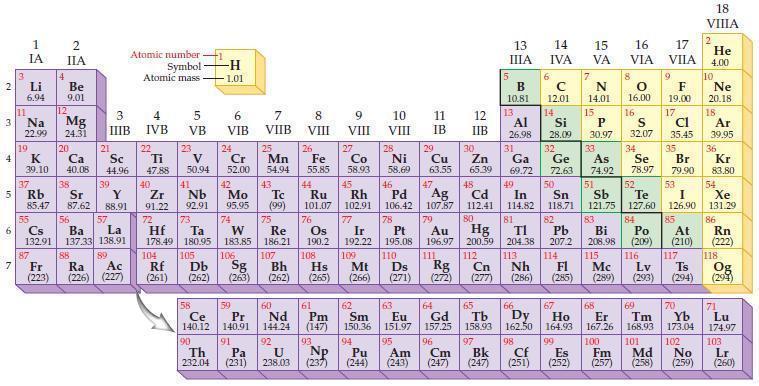
Use the periodic table to predict the ionic charge for each of the following nonmetal ions. (a)
Question:
Use the periodic table to predict the ionic charge for each of the following nonmetal ions.
(a) Cl ion
(b) I ion
(c) Se ion
(d) P ion.
Periodic Table
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 Al 26.98 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 Po (209) 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Here are the predicted ionic charges for each nonmetal ion based on the periodic table a Cl ion Chlo...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Use the periodic table to predict an ionic charge for each of the following metal ions. (a) Na ion (b) Ba ion (c) Ga ion (d) Pb ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55...
-
Use the periodic table to predict an ionic charge for each of the following metal ions. (a) Li ion (b) Mg ion (c) Al ion (d) Sn ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55...
-
Use the periodic table to predict the ionic charge for each of the following nonmetal ions. (a) F ion (b) Br ion (c) S ion (d) N ion. Periodic Table 2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55...
-
Ames Manufacturing Plant produces two different replacement parts for dishwashers. During a normal shift, they have 7 hours of time available on their main fabrication machine. The machine will...
-
Which unsecured claims have priority in a Chapter 7 liquidation case? Discuss in terms of priority ranks.
-
What are some potential explanations for why job satisfaction levels are on the rise, even in the face of seemingly stagnant drivers?
-
Describe project quality management (PQM) in terms of planning for quality, quality assurance, and quality control to continuously improve the projects products and supporting processes. AppendixLO1
-
Every time I think Ive captured user information requirements, theyve already changed. Its like trying to hit a moving target. Half the time, I dont think they even know what they want themselves,...
-
On December 31st of 2019, Stark Industries had assets of $4,562,309 and liabilities of $943,130. During 2020 their assets increased by $1,418,613 and their liabilities increased by $445,982. What was...
-
Draw the electron dot formula for HClO and determine if the molecule contains a coordinate covalent bond.
-
Based on electronegativity trends in the periodic table, which of the following molecules is most polar: HF, HCl, HBr, HI?
-
unoppolati
-
Use the following data to calculate the requested ratios for Tristar Transport and Logistic Services. Briefly analyse each answer. 1) Days accounts receivable. 2) Inventory turnover. 3) Debt/equity....
-
The following partial information is contained in the variance analysis received from the Western Plant of Eastlawn Company. All plants at Eastlawn apply overhead on the basis of direct labor-hours....
-
The Hudson Company is the sponsor of an IRS qualified defined benefit pension plan for a single employer. The pension plan calculates pension benefits based on factors like age, years of service, and...
-
Find the area enclosed by one loop of the four-leaved rose r = cos(20).
-
Landen Corporation uses a job-order costing system. At the beginning of the year, the company made the following estimates: Direct labor-hours required to support estimated production 65,000...
-
Typically there are two alternatives in a replacement analysis. One alternative is to replace the defender now. The other alternative is which one of the following? (a) Keep the defender for its...
-
Identify one local business that uses a perpetual inventory system and another that uses a periodic system. Interview an individual in each organization who is familiar with the inventory system and...
-
CVP analysis, margin of safety Suppose Latin Corp.s breakeven point is revenues of $1,500,000. Fixed costs are $600,000. 1. Compute the contribution margin percentage. 2. Compute the selling price if...
-
Operating leverage Color Rugs is holding a two-week carpet sale at Jerrys Club, a local warehouse store. Color Rugs plans to sell carpets for $500 each. The company will purchase the carpets from a...
-
CVP analysis, international cost structure differences Knitwear, Inc., is considering three countries for the sole manufacturing site of its new sweater: Singapore, Thailand, or the United States....
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


