Weather conditions affect the smog equilibrium in the atmosphere. What happens to the nitrogen dioxide concentration on
Question:
Weather conditions affect the smog equilibrium in the atmosphere. What happens to the nitrogen dioxide concentration on
(a) Hot, sunny days and
(b) Cool, overcast days?
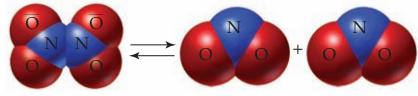
N2O4(g) + heat ⇄ 2 NO2(g)
Transcribed Image Text:
Ν Ν N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a On hot sunny days the temperature increases According to Le Chat...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The following table provides a 14-day record of weather conditions and whether or not golf was played on each day. (a) Compute the associated training set. (b) Determine the decision whether or not...
-
Explain why economists need to consider and set aside the value of depreciation in the determination of the countrys economic performance.
-
Restate the a priori property in your own words. For the following several exercises, consider the following data set from Quinlan [4] shown as Table 12.8. The goal is to develop association rules...
-
Examine the articles reproduced below and consider how the five C's discussed in the course have application in the present coronavirus pandemic. "To the extent that an environment characterized by...
-
Define each of the following terms: a. Multinational corporation b. Exchange rate; fixed exchange rate system; floating exchange rates c. Trade deficit; devaluation; revaluation d. Exchange rate...
-
If $100,000 will purchase a 20-year annuity paging $632.65 at each month's end, what monthly compounded nominal rate and effective rate of interest are earned by the funds?
-
Under what circumstances is it ethical to use consumer information in marketing research?
-
On April 1, Moloney Meat Distributors sold merchandise on account to Fronkes Franks for $3,000 on Invoice 1001, terms 1/10, n/30. Payment was received in full from Fronkes Franks, less discount, on...
-
Sweetwater Company manufactures two products, Mountain Mist and Valley Stream. The company prepares its master budget on the basis of standard costs. The following data are for March. Standards...
-
The industrial process for producing hydrogen gas involves reacting methane and steam at a high temperature. CH 4 (g) + H 2 O(g) + heat CO(g) + 3 H 2 (g) Predict the direction of equilibrium shift...
-
The conditions for producing ammonia industrially are 500 C and 300 atm. What happens to the ammonia concentration if (a) The temperature increases and (b) The pressure increases? N 2 (g) + 3 H 2 (g)...
-
Differentiate between the functions of governor and flywheel.
-
The following information is available for two different types of businesses for the 2011 accounting period. Dixon Consulting is a service business that provides consulting services to small...
-
Marino Basket Company had a \(\$ 6,200\) beginning balance in its Merchandise Inventory account. The following information regarding Marino's purchases and sales of inventory during its 2011...
-
On March 6, 2011, Bob's Imports purchased merchandise from Watches Inc. with a list price of \(\$ 31,000\), terms \(2 / 10, n / 45\). On March 10, Bob's returned merchandise to Watches Inc. for...
-
The following events apply to Tops Gift Shop for 2012, its first year of operation: 1. Acquired \(\$ 45,000\) cash from the issue of common stock. 2. Issued common stock to Kayla Taylor, one of the...
-
Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Transportation-in. b. Insurance on the office building. c. Office supplies. d. Costs...
-
Graphs 11a-c were produced by a recursive formula in the form u0 = a un= un - 1 (1 + p) + d where n ( 1 For each case, tell if a, p, and d are greater than zero (positive), equal to zero, or less...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
Three grams of musk oil are required for each bottle of Mink Caress, a very popular perfume made by a small company in western Siberia. The cost of the musk oil is 150 roubles per kilogram. (Siberia...
-
The production manager of Rordan Corporation has submitted the following of units to be produced by quarter for the upcoming fiscal year: Each unit requires 0.35 direct labor-hours, and direct...
-
The direct labor budget of Yuvwell Corporation fiscal year contains the following details concerning budgeted direct labor-hours: The companys variable manufacturing overhead rate is $3.25 per direct...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...
-
BE13.2 (LO 1), AP An inexperienced accountant for Silva Corporation showed the following in the income statement: net income \$337,500 and unrealized gain on availablefor-sale securities (before...

Study smarter with the SolutionInn App


