What is the mass in grams of 2.01 x 10 22 atoms of sulfur? Strategy Plan STEP
Question:
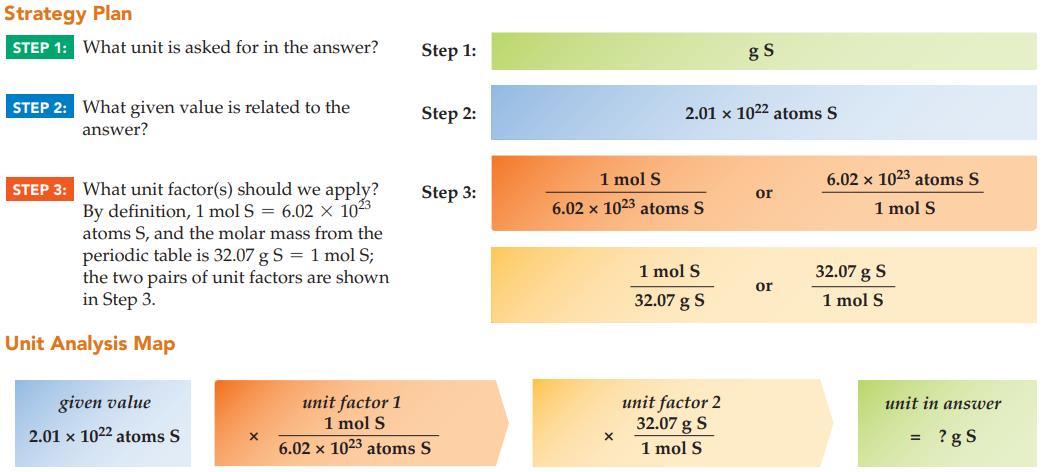
What is the mass in grams of 2.01 x 1022 atoms of sulfur?
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? By definition, 1 mol S = 6.02 x 1023 atoms S, and the molar mass from the periodic table is 32.07 g S = 1 mol S; the two pairs of unit factors are shown in Step 3. Unit Analysis Map given value 2.01 x 1022 atoms S X Step 1: Step 2: Step 3: unit factor 1 1 mol S 6.02 x 1023 atoms S 1 mol S 6.02 x 1023 atoms S X 2.01 x 1022 atoms S 1 mol S 32.07 g S gS unit factor 2 32.07 g S 1 mol S or or 6.02 x 1023 atoms S 1 mol S 32.07 g S 1 mol S unit in answer ?gs =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
We apply the unit factor 1 mol S602 x ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
What is the mass in grams of solute in? (a) 250 mL of 0.264 M H2O2 (b) 37.0 mL of 5.75 x 10-4 M benzoic acid (122 g / mol)
-
Aspartame is an artificial sweetener that is 160 times sweeter than sucrose (table sugar) when dissolved in water. It is marketed as NutraSweet. The molecular formula of aspartame is C 14 H 18 N 2 O...
-
What is a Java package, and what is its purpose?
-
Presented below is the partial bond discount amortization schedule for Bilder Corp. Bilder uses the effective-interest method of amortization. Instructions(a) Prepare the journal entry to record the...
-
A researcher claims that the number of homicide crimes in California by month is uniformly distributed. To test this claim, you randomly select 1800 homicides from a recent year and record the month...
-
How does a company that has transac tions in a foreign currency determine the amounts to report in the financial statements? LO9
-
Prepare general journal entries for the following transactions of Sustain Company. Use the following (partial) chart of accounts: Cash; Prepaid Insurance; Accounts Receivable; Furniture; Accounts...
-
1- Joe's Auto Shop has the following annual information: Gross Sales, $719,000; Net Sales, $694,000; Gross Profit, $454,000. What are the shops returns and allowances? 2- Heavenly Coffee Shop has the...
-
State the number of moles represented by each of the following. (a) 6.02 x 10 23 atoms of sulfur, S (b) 6.02 x 10 23 molecules of sulfur dioxide, SO 2 .
-
What is the number of molecules in 1.00 mol of a diatomic nonmetal?
-
Find the indicated quantities for f(x) = 3x2. (A) The average rate of change of fix) if x changes from 2 to 5. (B) The slope of the secant line through the points (2,f(2)) and (5,f(5)) on the graph...
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
Use the vector dot product to find the obtuse angle between two diagonals of a cube. What is the chemical significance of this angle?
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
Hanson Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,800,000 on March 1, $1,200,000 on June 1, and $3,000,000 on December...
-
Hanson Company (see BE10-2) borrowed $1,000,000 on March 1 on a 5-year, 12% note to help finance construction of the building. In addition, the company had outstanding all year a 10%, 5-year,...
-
Use the information for Hanson Company from BE10-2 and BE10-3. Compute avoidable interest for Hanson Company.
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


