Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) AgNO3(aq) + KI(aq) → AgI(s) + KNO3(aq)
(b) BaCl2(aq) + K2CrO4(aq) → BaCrO4(s) + KCl(aq)
Table 14.5
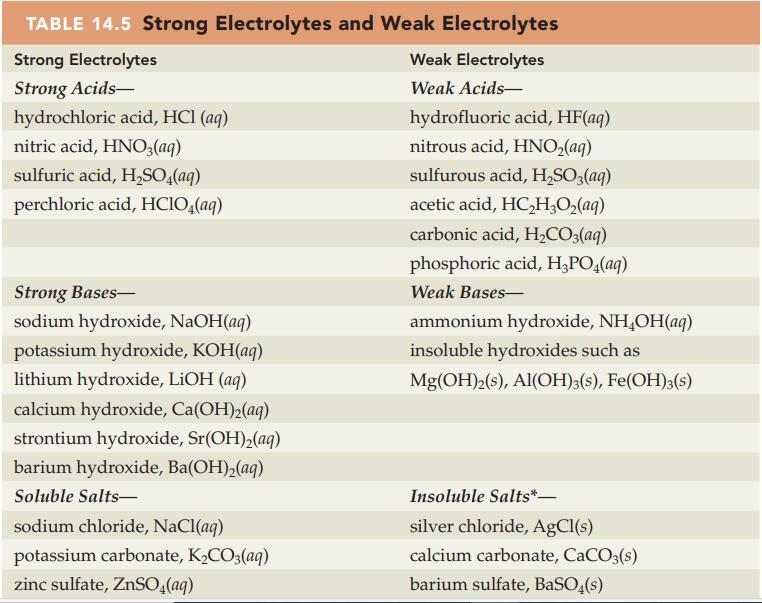
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Strong Acids- hydrochloric acid, HCl(aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) Weak Electrolytes Weak Acids- hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Ag aq I a...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) HF(aq) + Li 2 CO 3 (aq) LiF(aq) + H 2 O(l) + CO...
-
Find the exact value of sin(x - y) if sin(x) = 3T 3T T
-
The current price of a stock is $33, and the annual risk-free rate is 6%. A call option with a strike price of $32 and with 1 year until expiration has a current value of $6.56. What is the value of...
-
Determine the moment of inertia about the x axis. y = a
-
Service costing is adopted by (a) cinema houses (b) electricity companies (c) gas supply (d) all the above
-
New Wave Images is a graphics design firm that prepares its financial statements using a calendar year. Manny Kinn, the company treasurer and vice president of finance, has prepared a classified...
-
Current Attempt in Progress Novak Company began operations on January 1, 2019, adopting the conventional retail inventory system. None of the company's merchandise was marked down in 2019 and,...
-
A drop of methyl red and a drop of phenolphthalein are added to a beaker of distilled water. What is the resulting color of the water?
-
What term describes ions in a total ionic equation that do not react?
-
What is the purpose of the accept method of the ServerSocket class?
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
Write the lowest-degree polynomial function that has the given set of zeros and whose graph has the given y-intercept. a. zeros: x = - 4, x = 5, x = - 2 (double root); y-intercept: - 80 b. zeros: x =...
-
Synthesize the products by drawing out reagents and intermediates along the way. `N H. OH HO HO
-
Special Order Louisville Corporation produces baseball bats for kids that it sells for $32 each. At capacity, the company can produce 50,000 bats a year. The costs of producing and selling 50,000...
-
Contribution approach, relevant costs . Air Frisco has leased a single jet aircraft that it operates between San Francisco and the Fijian Islands. Only tourist-class seats are available on its...
-
Relevant costs, opportunity costs. Larry Miller, the general manager of Basil Software, must decide when to release the new version of Basils spreadsheet package, Easyspread 2.0. Development of...
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


