Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following acid–base reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) HF(aq) + Li2CO3(aq) → LiF(aq) + H2O(l) + CO2(g)
(b) H2SO4(aq) + Ba(OH)2(aq) → BaSO4(s) + H2O(l)
Table 14.5
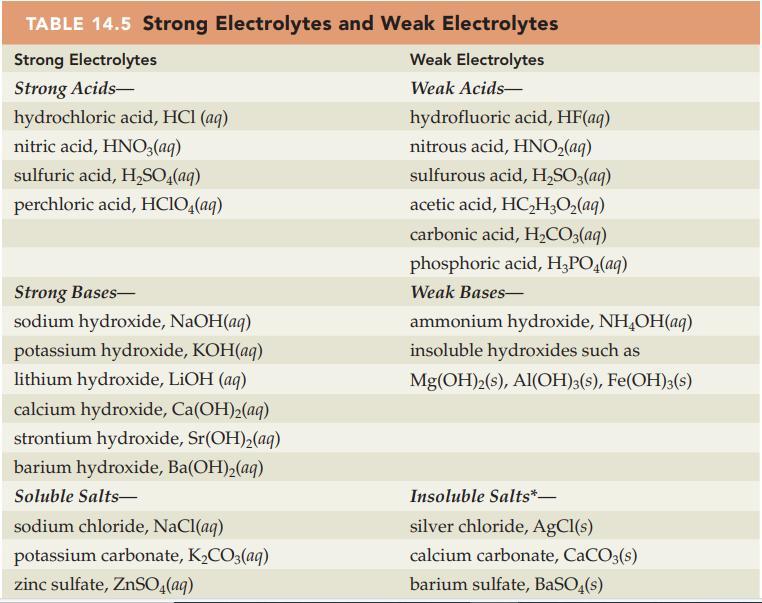
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Weak Electrolytes Strong Acids- Weak Acids- hydrochloric acid, HCl (aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Completed and balanced c...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) AgNO 3 (aq) + KI(aq) AgI(s) + KNO 3 (aq) (b)...
-
Consider an atom diffuses in a 3 D simple cubic lattice by a random walk mechanism. The atom jumps 6 x 1 0 - 5 times per second at 3 0 0 K and 3 x 1 0 4 times per second at 6 0 0 K . Assuming that...
-
The exercise price on one of Flanagan Companys options is $15, its exercise value is $22, and its time value is $5. What are the options market value and the price of the stock?
-
Based on your measurements and calculations (exercise 13), what is the compression ratio, CR, of the lawnmower engine? Refer to a 3.5 horsepower lawn mower engine at a nominal speed of 2.50 10 3...
-
Canteens apply (a) contract costing (b) job costing (c) service costing (d) batch costing
-
The following data are for Montgomery Retail Outlet Stores. The account balances (in thousands) are for 2014. Marketing and advertising costs ..........$ 48,000 Merchandise inventory, January 1, 2014...
-
As part of its stock - based compensation package, International Electronics ( IE ) granted 8 million stock appreciation rights ( SARs ) to t officers on January 1 , 2 0 2 4 . At exercise, holders of...
-
What term describes ions in a total ionic equation that do not react?
-
List the four steps for writing a balanced net ionic equation.
-
Simplify and check using a graphing calculator. 4 cosx sinx sin x 4 cos x, 2
-
Test the given claim. Assume that a simple random sample is selected from a normally distributed population. Use either the P-value method or the traditional method of testing hypotheses. Company A...
-
Trojan Technologies As Joyce Guo, senior buyer at Trojan Technologies Inc. in London, Ontario, Canada, finished her presentation, Randy Haill, materials manager, Made the following comments to her:...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
Express the confidence interval (0.045,0.123) in the form of p^ - E < p < p^+ E.
-
Boomtown is preparing a cost analysis of the three departments: Parks. Fire, and Water. To comply with accuracy standards in allocating indirect costs, Boomtown will employ the step-down method of...
-
The graph of y = 2(x - 3)(x - 5)(x + 4)2 has x-intercepts 3, 5, and - 4 because they are the only possible x-values that make y = 0. This is a 4th-degree polynomial, but it has only three...
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
Make or buy unknown level of volume. (A. Atkinson) Oxford Engineering manufactures small engines. The engines are sold to manufacturers who install them in such products as lawn mowers. The company...
-
Make versus buy, activity-based costing opportunity costs. (N. Melumad and S. Reichelstein, adapted) The Ace Company produces bicycles. This years expected production is 10,000 units. Currently, Ace...
-
Multiple choice comprehensive problem on relevant costs. The following are the Class Companys unit costs of manufacturing and marketing a high-style pen at an output level of 20,000 units per month:...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


