Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following acid–base reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)
(b) HC2H3O2(aq) + Ca(OH)2(aq) → Ca(C2H3O2)2(aq) + H2O(l)
Table 14.5
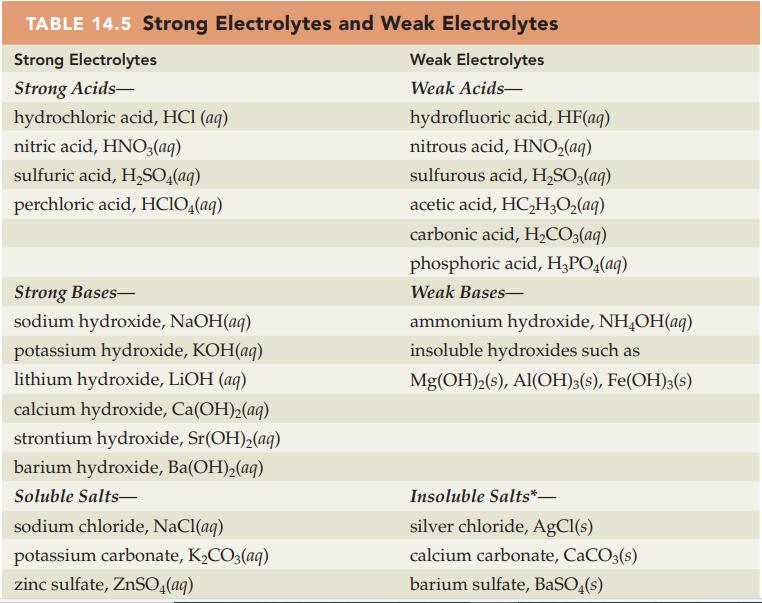
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Weak Electrolytes Strong Acids- Weak Acids- hydrochloric acid, HCl (aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a H aq OH aq H ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) HF(aq) + Li 2 CO 3 (aq) LiF(aq) + H 2 O(l) + CO...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Which of the following statements about close buyer-seller relationships in business markets is FALSE? Long-term commitments on larger order quantities often cause the supplier to raise its selling...
-
Describe the effect on a call options price that results from an increase in each of the following factors: (1) Stock price, (2) Strike price, (3) Time to expiration, (4) Risk-free rate, and (5)...
-
Determine the Diesel cycle efficiency of an engine that has a compression ratio of 18.6 and a cut-off ratio of 2.2.
-
In hospitals, the cost unit is (a) per bed (b) per tablet (c) per doctor (d) per patient
-
Contrast sources and uses of cash referencing using at least two examples of assets and liabilities (four totals). Provide examples of how cash is used or provided depending on whether it is...
-
Identifying and Analyzing Financial Statement Effects of Dividends The stockholders' equity of DiFrancesco Company at March 31, 2019 is shown below. 4% preferred stock, $1,000 par value, 25,000...
-
List the four steps for writing a balanced net ionic equation.
-
Write the following salts in either the ionized or the nonionized form to best represent an aqueous solution. (a) AlPO 4 (s) (b) Co(C 2 H 3 O 2 ) 3 (aq) (c) MnSO 4 (aq) (d) PbSO 4 (s).
-
What are three different ways preventive maintenance is scheduled?
-
The waiting times between a subway departure schedule and the arrival of a passenger are uniformly distributed between 0 and 9 minutes. Find the probability that a randomly selected passenger has a...
-
Greenview Dairies produces a line of organic yogurts for sale at supermarkets and specialty markets in the Southeast. Economic conditions and changing tastes have resulted in slowing demand growth....
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Inc. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2019, are as indicated. Described here are...
-
If you were team leader how would you break up this assignment for 4 people to complete? Group Case Analysis Parts 4, 5, and 6 IV. STRATEGY IMPLEMENTATION. (How are you going to do what you want to...
-
A genetic experiment with peas resulted in one sample of offspring that consisted of 440 green peas and 166 yellow peas. Construct a 90% confidence interval to estimate of the percentage of yellow...
-
Write the factored form for each polynomial function. Check your work by graphing on your calculator. a. b. c. d. -intercept: 103 r-intereept: 108 -50 1sp -intereept:-100 -inlercept:-90
-
Fred Farmer needs to prepare a balance sheet for his bank. He spent the day getting the following information. Fred needs your help to build a balance sheet and evaluate it. The information was...
-
Make versus buy, ethics. (CMA, adapted) Lynn Hart is a management accountant at Paibec Corporation. Paibec is under intense cost competition. Hart has been asked to evaluate whether Paibec should...
-
Product mix, constrained resource. Taylor Furniture produces and sells specialty mattresses. Production is a machine-intensive process. Taylors variable costs are direct material costs, variable...
-
Normal and abnormal spoilage in units. The following data, in physical units, describe a grinding process for January: Inspection occurs at the 100% completion stage. Normal spoilage is 5% of the...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


