Write the equilibrium constant expression for each of the following weak bases: (a) NHOH(aq) (b) CH5NH(aq) (c)
Question:
Write the equilibrium constant expression for each of the following weak bases:
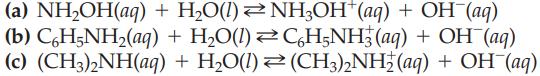
Transcribed Image Text:
(a) NH₂OH(aq) (b) CáH5NH₂(aq) (c) (CH3)2NH(aq) + H₂O(1) + H₂O(1) + NH3OH*(aq) + OH-(aq) CáH5NH3(aq) + OH(aq) H₂O(l)(CH3)2NH2 (aq) + OH¯(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The equilibrium constant expression K for a weak base ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write the equilibrium constant expression for each of the following weak acids: (a) HCHO(aq) H(aq) + CHO (aq) (b) H,C,O4(aq)H*(aq) + HC,O4 (aq) (c) HC6H5O7(aq) H*(aq) + HCHO7 (aq)
-
The following equilibrium constants were determined at 1123 K: Write the equilibrium constant expression KP, and calculate the equilibrium constant at 1123 K for C(s) + CO2(g)--2CO(g) CO(g) + Cl2(g)...
-
Write the equilibrium constant expression for the following weak acid: H 2 CO 3 (aq) H + (aq) + HCO 3 (aq)
-
Which of the following statements about Erlang are TRUE? Check all that apply All functions in an .erl file are public by default, i.e. callable from the shell or from any other file All parameters...
-
What is a Eurodollar? If a French citizen deposits $10,000 in Chase Bank in New York, have Eurodollars been created? What if the deposit is made in Barclays Bank in London? Chases Paris branch? Does...
-
Track the future values of two investments of $5000, one at 6.3% compounded quarterly and another at 6.3% compounded monthly for each interest payment period for 10 years. (a) How long does it take...
-
What is the difference between internal and external secondary research?
-
Saratoga Ltd. was having difculty in raising nance for expansion. Kingsh Ltd. was interested in achieving economies by marketing a wider range of products. The following shows the nancial positions...
-
Concerning the decision to keep or drop a product line, a product line contributes $100,000 toward unavoidable costs of $140,000. (That is, revenues less all avoidable costs equals a positive...
-
Nitrous acid, HNO 2 , is used in the synthesis of organic dye compounds. If the hydrogen ion concentration of a 0.125 M solution is 7.5 x 10 -3 M, what is the ionization constant for the acid?
-
Smog contains formaldehyde that is responsible for an eye burning sensation. Formaldehyde, CH 2 O, is produced from the reaction of ozone and atmospheric ethylene, C 2 H 4 , as follows. 2 C 2 H 4 (g)...
-
A passive solar house that is losing heat to the outdoors at 5C at an average rate of 50,000 kJ/h is maintained at 22C at all times during a winter night for 10 h. The house is to be heated by 50...
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
Solve. a. log 35 + log 7 = log x b. log 500 - log 25 = log x c. log (1/8 = x log8 d. 15(9.4)x = 37000 e. f. log6 342 = 2x
-
What tools are available to help shoppers compare prices, features, and values and check other shoppers opinions?
-
What is a budget? What is budgetary control?
-
Discuss some of the major benefits to be gained from budgeting.
-
What is meant by the term responsibility accounting?
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


