Write the equilibrium constant expression for each of the following weak acids: (a) HCHO(aq) H(aq) +
Question:
Write the equilibrium constant expression for each of the following weak acids:
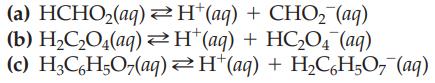
Transcribed Image Text:
(a) HCHO₂(aq) → H(aq) + CHO₂ (aq) (b) H,C,O4(aq)≥H*(aq) + HC,O4 (aq) (c) H₂C6H5O7(aq) H*(aq) + H₂C₂H₂O7¯ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a K b K c ...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write the equilibrium constant expression for each of the following weak bases: (a) NHOH(aq) (b) CH5NH(aq) (c) (CH3)2NH(aq) + HO(1) + HO(1) + NH3OH*(aq) + OH-(aq) CH5NH3(aq) + OH(aq) HO(l)(CH3)2NH2...
-
The following equilibrium constants were determined at 1123 K: Write the equilibrium constant expression KP, and calculate the equilibrium constant at 1123 K for C(s) + CO2(g)--2CO(g) CO(g) + Cl2(g)...
-
Write the equilibrium constant expression for the following weak acid: H 2 CO 3 (aq) H + (aq) + HCO 3 (aq)
-
Jake, a single taxpayer, has $100,000 of ordinary income, a $10,000 net short-term capital loss, and $7,000 of qualified dividends. What is the result?
-
Why do U.S. corporations build manufacturing plants abroad when they could build them at home?
-
A deferred annuity is comprised of eight annual payments of $1500. What is the period of deferral if the present value of the payments, discounted at 4.9% compounded annually, is $8037.87?
-
What are the types of quantitative research?
-
The Friendly Sausage Factory (FSF) can produce hot dogs at a rate of 5,000 per day. FSF supplies hot dogs to local restaurants at a steady rate of 250 per day. The cost to prepare the equipment for...
-
Please solve the question using EXCEL and provide the used formulas in solving it :) For an optimum recovery of an oil field, two enhanced oil recovery (EOR) techniques. namely waterflooding and...
-
Smog contains formaldehyde that is responsible for an eye burning sensation. Formaldehyde, CH 2 O, is produced from the reaction of ozone and atmospheric ethylene, C 2 H 4 , as follows. 2 C 2 H 4 (g)...
-
The industrial process for producing hydrogen gas involves reacting methane and steam at a high temperature. CH 4 (g) + H 2 O(g) + heat CO(g) + 3 H 2 (g) Predict the direction of equilibrium shift...
-
Case 2. To remain competitive. SunSystems' management believes the company must produce Job B-type servers (from Decision Case 1) at a target cost of $5.400. SunSystems has just joined a B2B e-market...
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
Last semester, all of Ms. Nolte's students did projects. One-half of the students in her second-period class investigated fractals, one-fourth of the students in that class did research projects, and...
-
What is the role of business risk analysis in the audit planning process?
-
Describe the flow of budget data in an organization. Who are the participants in the budgeting process, and how do they participate?
-
What is a self-imposed budget? What are the major advantages of self-imposed budgets? What caution must be exercised in their use?
-
How can budgeting assist a company in planning its workforce staffing levels?
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


