Write the ionic charge for each of the following ions as predicted from the group number in
Question:
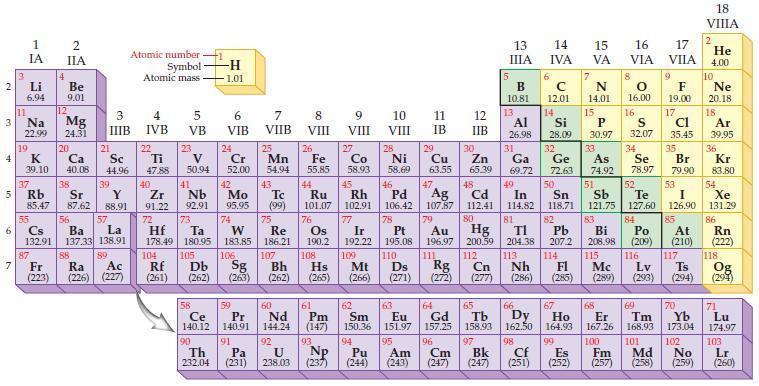
Write the ionic charge for each of the following ions as predicted from the group number in the periodic table.
(a) Be ion
(b) Sn ion
(c) P ion
(d) S ion.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 Sr 85.47 87.62 20 38 Ca Sc 40.08 44.96 21 56 Cs La Ba 132.91 137.33 138.91 88 3 IIIB 39 Y 88.91 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 91 7 VIIB Hf Ta W Re 178.49 180.95 183.85 186.21 Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 9 VIII 61 Pm (147) 27 108 Bh Mt Hs (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB 12 IIB 13 IIIA 5 B 10.81 13 Al 26.98 14 15 IVA 6 C 12.01 14 Si 28.09 32 17 16 VA VIA VIIA 8 7 N 14.01 15 P 30.97 79 Pt 195.08 110 Ds 81 82 Au Hg TI Pb 196.97 200.59 204.38 207.2 111 112 113 114 Rg Cn Nh Fl (271) (272) (277) (286) (285) 29 30 31 33 As Ge Se Cu Zn Ga 63.55 65.39 69.72 72.63 74.92 78.97 16.00 16 S 32.07 34 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 83 Bi 208.98 115 84 Po (209) 9 F 19.00 Md (258) 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 116 Mc Ts Lv (289) (293) (294) 66 67 70 63 64 65 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (4 reviews)
a b c d Beryllium Be is a member of groupIIA and has only ...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write the ionic charge for each of the following ions as predicted from the group number in the periodic table. (a) Cs ion (b) Ga ion (c) O ion (d) I ion. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1...
-
Predict the ionic charge for a sodium ion, Na ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 5 6 7 Li 6.94 11 Na 22.99 19 1 IA K 39.10 37 55 4 2 IIA 87 Be 9.01...
-
Predict the ionic charge for an aluminum ion, Al ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 Rb R 4 87 2 IIA Be...
-
In your own words, define or explain the terms or symbols (a) (b) [ ]; (c) Spectator ion; (d) Weak acid.
-
Multiple Choice. Choose the best answer. 1. Special purpose governments differ from general purpose governments in that special purpose governments: a. Provide a single function or limited range of...
-
As a genetics historian, you are repeating some of the classic experiments conducted by Jacob and Monod with the lactose operon in E. coli. You use an F plasmid to construct several E. coli strains...
-
A charity organization is selling $5 raffle tickets as part of a fund-raising program. The first prize is a trip to Mexico valued at $3450, and the second prize is a weekend spa package valued at...
-
The following information is available for Laurel Company, a wholesale company: Expected sales volume: October . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
Question 4 4 pts The Honda Motor Shop produces engine parts. Typically, 100 pieces out of a job lot of 2.000 parts are spoiled. Costs are assigned at the inspection point, $75.00 per unit. Spoiled...
-
Refer to the periodic table and predict which of the following ions are isoelectronic with the noble gas argon. (a) Al 3 + (b) Ca 2 + (c) S 2 (d) N 3 . Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA...
-
State the predicted ionic charge of nonmetal ions in each of the following groups of elements. (a) Group IVA/14 (b) Group VA/15 (c) Group VIA/16 (d) Group VIIA/17.
-
a. What is meant by self-selected sample? b. Give an example of a recent poll that involved a self-selected sample. c. Why are self-selected samples not desirable?
-
Question 2: Response to John Ripley?
-
Prepare a summary of the effects of the "fat tax" on the demand and supply diagram. Ensure you talk about the dead weight loss. PRICE D Tax Revenue S P1 P2 TAX P3 Dead Weight Loss QUANTITY QeAT QeBT...
-
Trade causes production in Home to move from point A to point B. What does this mean happened to the relative price of Qc? QF Home QF1 A QF2 Qc Qc2 Qc2
-
Write as an ordered pair the coordinates of the point whose y-coordinate is 3 and whose x-coordinate is -7. X Viewing Saved Work Revert to Last Response
-
1. A cereal manufacturer tests their equipment weekly to be assured that the proper amount of cereal is in each box of cereal. The company wants to see if the amount differs from the stated amount on...
-
How does a partner's share of partnership liabilities affect his or her outside basis?
-
Use the following data to answer the next two (2) questions: Product 1 Product 2 Product 3 Direct Material Cost $25,000 $30,000 $35,000 Direct Labor Cost $30,000 $40,000 $50,000 Direct Labor Hours...
-
(EPS with Convertible Bonds) On June 1, 2009, Bluhm Company and Amanar Company merged to form Davenport Inc. A total of 800,000 shares were issued to complete the merger. The new corporation reports...
-
(EPS with Convertible Bonds and Preferred Stock) The Ottey Corporation issued 10-year, $4,000,000 par, 7% callable convertible subordinated debentures on January 2, 2010. The bonds have a par value...
-
(EPS with Convertible Bonds and Preferred Stock) On January 1, 2010, Lindsey Company issued 10-year, $3,000,000 face value, 6% bonds, at par. Each $1,000 bond is convertible into 15 shares of Lindsey...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


