The entropy change to bring a sample from 0 K (absolute zero) to a given state is
Question:
The entropy change to bring a sample from 0 K (absolute zero) to a given state is called the absolute entropy of the sample in that state.
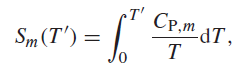
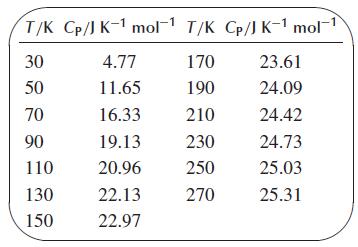
where Sm(T′) is the absolute molar entropy at temperature T′,CP,m is the molar heat capacity at constant pressure, and T is the absolute temperature. Using Simpson’s rule, calculate the absolute entropy of 1.000 mol of solid silver at 270 K. For the region 0–30 K, use the approximate relation Cp = aT3, where a is a constant that you can evaluate from the value of Cp at 30 K. For the region 30–270 K, use the following data:3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





