When a gas expands reversibly, the work that it does on its surroundings is given by the
Question:
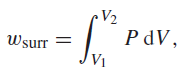
where V1 is the initial volume, V2 is the final volume, and P is the pressure of the gas. Certain nonideal gases are described by the van der Waals equation of state,
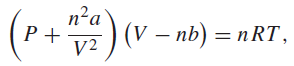
where V is the volume, n is the amount of gas in moles, T is the temperature on the Kelvin scale, and a and b are constants. R is usually taken to be the ideal gas constant, 8.3145 J Kˆ’1 molˆ’1.
(a) Obtain a formula for the work done on the surroundings if 1.000 mol of such a gas expands reversibly at constant temperature from a volume V1 to a volume V2.
(b) If T = 298.15 K,V1 = 1.00l (1.000 × 10ˆ’3 m3), and V2 = 100.0l = 0.100 m3, find the value of the work done for 1.000 mol of CO2, which has a = 0.3640 Pa m6 molˆ’2 and b = 4.267 × 10ˆ’5 m3 molˆ’1. The ideal gas constant, R = 8.3145 J Kˆ’1 molˆ’1.
(c) Calculate the work done in the process of part b if the gas is assumed to be ideal.
Step by Step Answer:






