Question: A model hydrogen atom is composed of a point nucleus with charge +|e| and an electron charge distribution Show that the ionization energy (the energy
A model hydrogen atom is composed of a point nucleus with charge +|e| and an electron charge distribution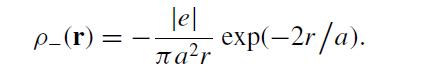
Show that the ionization energy (the energy to remove the electronic charge and disperse it to infinity) of this atom is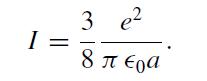
p_(r) = lel ?, exp(-2r/a). :
Step by Step Solution
3.27 Rating (165 Votes )
There are 3 Steps involved in it
The total charge and potential have contributions f... View full answer

Get step-by-step solutions from verified subject matter experts


