A chemical dissolves in water at a rate jointly proportional to the amount undissolved and to the
Question:
A chemical dissolves in water at a rate jointly proportional to the amount undissolved and to the difference between the concentration in the solution and that in the saturated solution. Initially none of the chemical is dissolved in the water. Show that the amount x(t) of undissolved chemical satisfies the differential equation
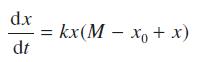
where k is a constant, M is the amount of the chemical in the saturated solution and x0 = x(0).
Transcribed Image Text:
dx dt = kx(M - xo + x)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Amount undissolved is x at time t Amount i...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A curved bar ABC is subjected to loads in the form of two equal and opposite forces P, as shown in the figure. The axis of the bar forms a semicircle of radius r. Determine the axial force N, shear...
-
In the circuit shown in Fig all the resistors are rated at a maximum power of 1.00 W. What is the maximum emf e that the battery can have without burning up any of the resistors? 25.0 1 25.0n 30.00...
-
Suppose the time between the arrivals of successive cars at a toll booth is measured by a random variable X that is exponentially distributed with the density function where x measures time (in...
-
In Exercises show that the function y = (x) is a solution of the differential equation. y = 4e-x y" - y = 0
-
A beam of hydrogen molecules (H2) is directed toward a wall, at an angle of 55o with the normal to the wall. Each molecule in the beam has a speed of 1.0 km/s and a mass of 3.3 x 10-24 g. The beam...
-
A financial firm holds a bond in its investment portfolio whose duration is 15 years. Its current market price is $975. While market interest rates are currently at 6 percent for comparable quality...
-
True or False: The probability that the uniform random variable will assume a value in any interval of equal length is different for each interval. LO8
-
Trisha, whose tax rate is 35%, sells the following capital assets in 2015 with gains and losses as shown: a. Determine Trishas increase in tax liability as a result of the three sales. All assets are...
-
Following is the information in relation to the defined benefit pension plan of Mimoza Ltd. for the year 2020. IFRS is the applicable standard here: DBO, Jan 1 $375,000 Fair value of plan assets, Jan...
-
The rate at which a solute diffuses through a membrane is proportional to the area and to the concentration difference across the membrane. A solution of concentration C flows down a tube with...
-
The limiting tension in a rope wound round a capstan (that is, the tension when the rope is about to slip) depends on the angle of wrap , as shown in Figure 8.15. Show that an increase in the angle...
-
Turner Corporation uses the calendar year as its tax year. It purchases and places into service $1.97 million of property during 2017 to use in its business: What is Turners total depreciation...
-
3. The Balance Sheet of International Operators Ltd. as at 31.03.2021 disclose the following position: PARTICULARS SHARE CAPITAL RESERVES AND SURPLUS SECURED LOANS UNSECURED LOANS CURRENT LIABILITY...
-
A uniformly charged ring of radius a. (a) The field at P on the x axis due to an element of charge dq. (b) The perpendicular component of the field at P due to segment 1 is canceled by the...
-
At what rate would $1,000 have to be invested to grow to $4,046 in 10 years?
-
Add F1 and F2 using graphical method, (triangle or parallelogram) Determine: 1 Magnitude,2. Direction measured CCW from positive axis, im now to America need help. CoursHeroTranscribedText 20 F-SON...
-
What is Monetary Policy? What is Monetary Base or High Powered Money? How commercial Banks create money Supply? Hint: By giving loans through creating checking account What is deposit multiplier?...
-
Finding equilibria of nonlinear coupled differential equations requires solving nonlinear simultaneous equations that can have any number of solutions. For each of the following pairs, solve each...
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
Draw the form of l-glutamic acid that predominates at each pH: (a) 1.9 (b) 2.4 (c) 5.8 (d) 10.4
-
For each of the following amino acids, draw the form that is expected to predominate at physiological pH: (a) l-Isoleucine (b) l-Tryptophan (c) l-Glutamine (d) l-Glutamic acid
-
Using the data in the following table, calculate the pI of the following amino acids: (a) l-Alanine (b) l-Asparagine (c) l-Histidine (d) l-Glutamic acid THE PK,VALUES FOR TWENTY NATURALLY OCCURRING...
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....
-
This is very confusing please help with descriptions if possible. Complete this question by entering your answers in the tabs below. Prepare a master budget for the three-month period ending June 30...
-
Doug recibe un dplex como regalo de su to. La base del to para el dplex y el terreno es de $90,000. En el momento de la donacin, el terreno y el edificio tienen un FMV de $40 000 y $80 000,...

Study smarter with the SolutionInn App


