Find the maximum or minimum values of the quadratic functions given in Question 26. Data from Question
Question:
Find the maximum or minimum values of the quadratic functions given in Question 26.
Data from Question 26
Determine which of the following quadratic functions are irreducible.
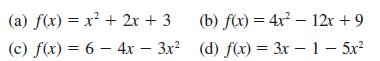
Transcribed Image Text:
(a) f(x) = x + 2x + 3 (c) f(x) = 6 - 4x - 3x (b) f(x) = 4x 12x + 9 (d) f(x) = 3x - 1 - 5x -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a Minimum value 2 attained at ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A uniform disk with mass m = 8.93 kg and radius R = 1.32 m lies in the x-y plane and centered at the origin. Three forces act in the +y-direction on the disk: 1) a force 343 N at the edge of the disk...
-
Use Lagrange multipliers to find the maximum or minimum values of f(x, y) subject to the constraint. f(x, y) = 3x 2y, x 2 + 2y 2 = 44
-
Use Lagrange multipliers to find the maximum or minimum values of f(x, y) subject to the constraint. f(x, y) = 5xy, x + 3y = 24
-
Campione Manufacturing acquired an 80% interest in DaLuca Distributors, a foreign corporation established on November 1, 2006, for 650,000 foreign currency units (FC). Campione acquired its 80%...
-
Use Norton?s theorem to find I o in the circuit in figure. 12 V 4 kn 2 kn +) 2 kn3 2 kn 4 kn 4 mA
-
A major utility company is worried about the project managers upgrading functional employees. On an eight-month project that employs four hundred full-time project employees, the department managers...
-
What control systems do you believe are linked to productivity and effectiveness of workers in Japan, the United States, Russia and South Africa? Please support your answer with Internet or other...
-
1. If Amanda and David wanted to transfer the ownership of all 5 policies to Paws and Claws today through an absolute assignment, calculate the total amount of their donation receipt they would be...
-
A defined benefit pension plan expects to pay out $20 million per year over the next 18 years to pensioners. The fund currently has $168 million in pension assets that are earning 5.2% per year. By...
-
Obtain the expansion about x = 2 of the function y = x 3 3x 2 + 6x 4.
-
Show that f(x) = x 3 3x 2 + 6x 4 is zero at x = 1, and hence factorize f(x).
-
Consider the problems related to controlling discretionary cost.
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
Predict the products from the following reactions. (a) (b) (c) (d) (1) 2 exces) NaoH, Ho (2) H,o KOH 2 HOEIOH KOH H2O/EtOH (1) LDA (1.1 equiv) (3) H2o
-
Anna, a high school counselor, devised a program that integrates classroom learning with vocational training to help adolescents at risk for school dropouts stay in school and transition to work...
-
A cylindrical vessel with rigid adiabatic walls is separated into two parts by a frictionless adiabatic piston. Each part contains 45.0 L of an ideal monatomic gas with C V ,m = 3/2R. Initially, T i...
-
Compound A has molecular formula C 5 H 12 and undergoes monochlorination to produce four different constitutional isomers. (a) Draw the structure of compound A. (b) Draw all four monochlorination...
-
Predict the stereochemical outcome of radical bromination of the following alkanes: (a) (b) (c) (d)
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


