An H 2 in its ground electronic, vibrational, and rotational state absorbs a photon of frequency 1.356
Question:
An H2 in its ground electronic, vibrational, and rotational state absorbs a photon of frequency 1.356 x 1014 Hz, undergoing a transition to the v = 1, ℓ = 1 state while remaining in the electronic ground state. It then undergoes a transition to the v = 0, ℓ = 2 state, emitting a photon of frequency 1.246 x 1014 Hz.
(a) Compute the moment of inertia of the H2 molecule about an axis through the center of mass.
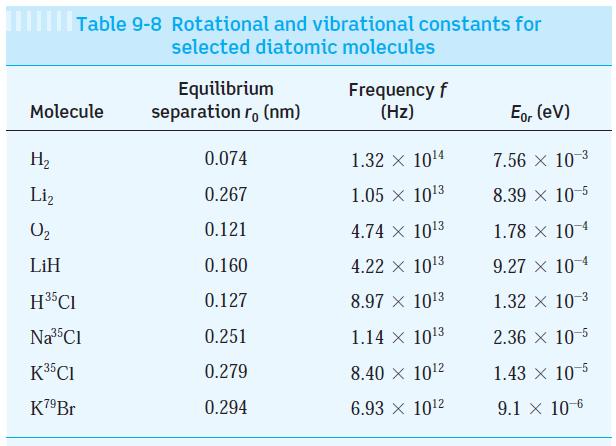
(b) Determine the vibrational frequency and r0 for H2 and compare these with the values in Table 9-8.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





