In this problem you are to obtain the Bohr results for the energy levels in hydrogen without
Question:
In this problem you are to obtain the Bohr results for the energy levels in hydrogen without using the quantization condition of Equation 4-17. In order to relate Equation 4-14
to the Balmer-Ritz formula, assume that the radii of allowed orbits are given by rn = n2r0, where n is an integer and r0 is a constant to be determined.
(a) Show that the frequency of radiation for a transition to nf = n - 1 is given by f ≈ kZe2/hr0n3 for large n.
(b) Show that the frequency of revolution is given by
![]()
(c) Use the correspondence principle to determine r0 and compare with Equation 4-19.
![]()
![]()
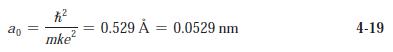
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





