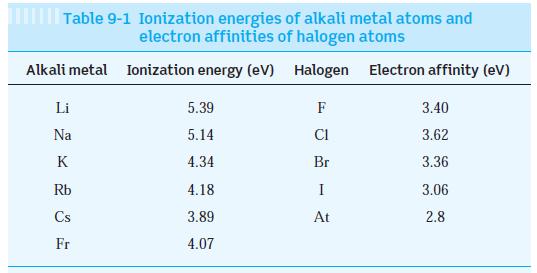
The observed dissociation energy of solid LiBr is 788 kJ/mol. Compute the cohesive energy of LiBr and
Question:
The observed dissociation energy of solid LiBr is 788 kJ/mol. Compute the cohesive energy of LiBr and compare the result with the value in Table 10-1. (Ionization energies for Li and Br are in Table 9-1.)
Table 10-1
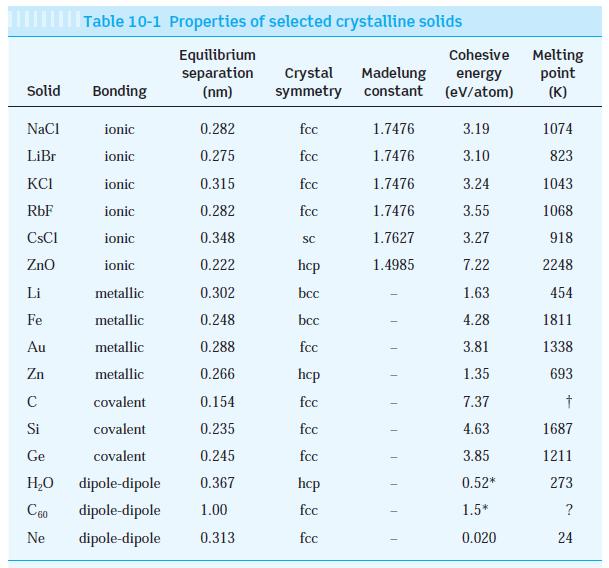
Table 9-1
Transcribed Image Text:
Solid NaCl LiBr KCI RbF ionic 0.282 ionic 0.275 ionic 0.315 ionic 0.282 ionic 0.348 ionic 0.222 metallic 0.302 metallic 0.248 metallic 0.288 metallic 0.266 covalent 0.154 covalent 0.235 Ge covalent 0.245 H₂O dipole-dipole 0.367 C60 dipole-dipole 1.00 Ne dipole-dipole 0.313 CsCl ZnO Table 10-1 Properties of selected crystalline solids Equilibrium separation (nm) Li Fe Au Zn C Si Bonding Crystal Madelung symmetry constant fcc fcc fcc fcc SC hcp bcc bcc fcc hcp fcc fcc fcc hcp fcc fcc 1.7476 1.7476 1.7476 1.7476 1.7627 1.4985 T 1 T Cohesive Melting energy (eV/atom) point (K) 3.19 3.10 3.24 3.55 3.27 7.22 1.63 4.28 3.81 1.35 7.37 4.63 3.85 0.52* 1.5* 0.020 1074 823 1043 1068 918 2248 454 1811 1338 693 † 1687 1211 273 ? 24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Cohesive energy LiBr This is ...View the full answer

Answered By

John Aketch
I have a 10 years tutoring experience and I have helped thousands of students to accomplish their educational endeavors globally. What interests me most is when I see my students being succeeding in their classwork. I am confident that I will bring a great change to thins organization if granted the opportunity. Thanks
5.00+
8+ Reviews
18+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
The ionization energies of sodium (in kJ/mol), starting with the first and ending with the eleventh, are 495.9, 4560, 6900, 9540, 13,400, 16,600, 20,120, 25,490, 28,930, 141,360, 170,000. Plot the...
-
The successive ionization energies for an unknown element are I1 = 896 kJ/mol I2 = 1752 kJ/mol I3 = 14,807 kJ/mol I4 = 17,948 kJ/mol To which family in the periodic table does the unknown element...
-
The first ionization energies of As and Se are 0.947 MJ/ mol and 0.941 MJ/mol, respectively. Rationalize these values in terms of electron configurations.
-
A 35 ft3 rigid tank has air at 225 psia and ambient 600 R connected by a valve to a piston cylinder. The piston of area 1 ft2 requires 40 psia below it to float, Fig. P3.99. The valve is opened and...
-
For Exercises, write the distribution for the formula and determine whether it is a probability distribution. P(X) = X / (X + 2) for X = 0, 1, 2
-
Suppose the same company wants to use bots to automate these tasks. How should the company go about planning for this change in inputs?
-
Suppose you fit a least squares line to 12 data points and the calculated value of SSE is .429. a. Find s2, the estimator of s2 (the variance of the random LO9 error term e). b. Find s, the estimate...
-
Paula Judge owns Judge Creative Designs. The trial balance of the firm for January 31, 2019, the first month of operations, is shown below. INSTRUCTIONS Complete the worksheet for the month. Prepare...
-
DeBole CPAs are currently completing the audit of McKeown Enterprises, a regional distributor of sheet metal and other building supplies in the Midwestern United States. The CPA firm has noted that...
-
Develop a database to help "YourPerfectEvent" event planners to manage their operations. The database must be able to accommodate the following. A customer contacts the company and makes...
-
(a) Using = 0.37 nm and (v) = 1.08 x 10 5 m/s at T = 300 K, calculate and for copper from Equations 10-13. Using the same value of , find and at (b) T = 200 K and (c) T = 100 K. me(v) nex and ...
-
The crystal structure of KCl is the same as that of NaCl. (a) Calculate the electrostatic potential energy of attraction of KCl, assuming that r 0 is 0.314 nm. (b) Assuming that n = 9 in Equation...
-
Anticipated consumer demand in a restaurant for free-range steaks next month can be modeled by a normal random variable with mean 1,200 pounds and standard deviation 100 pounds. a. What is the...
-
a) Describe the following concepts in the context of organizational development. b) Discuss how these concepts interrelate and support each other within an organizational framework
-
Q2. a) Analyze the importance of communication in the change management process. b) Suggests strategies that a Disaster Management Organization can employ to ensure effective communication during...
-
Q3. a) Explain the following Change Management Models
-
Q3. b) Discuss how each model can be applied in real-world organizational change scenarios.
-
In this question, you will work step-by-step through an optimization problem. A craftsman wants to make a cylindrical jewelry box that has volume, V, equal to 55 cubic inches. He will make the base...
-
Why isnt flash RAM commonly used to implement primary storage?
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Determine the signs (positive, negative, or zero) of the position, velocity, and acceleration for the particle in FIGURE Q1.8. Figure Q1.8
-
FIGURE EX1.9 shows five points of a motion diagram. Use Tactics Box 1.3 to find the average acceleration vectors at points 1, 2, and 3. Draw the completed motion diagram showing velocity vectors and...
-
FIGURE EX1.9 shows five points of a motion diagram. Use Tactics Box 1.3 to find the average acceleration vectors at points 1, 2, and 3. Draw the completed motion diagram showing velocity vectors and...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


