The crystal structure of KCl is the same as that of NaCl. (a) Calculate the electrostatic potential
Question:
The crystal structure of KCl is the same as that of NaCl.
(a) Calculate the electrostatic potential energy of attraction of KCl, assuming that r0 is 0.314 nm.
(b) Assuming that n = 9 in Equation 10-6, calculate the dissociation energy in eV per ion pair and in kcal/mol.
(c) The measured dissociation energy is 165.5 kcal/mol. Use this to determine n in Equation 10-6.
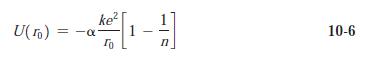
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





