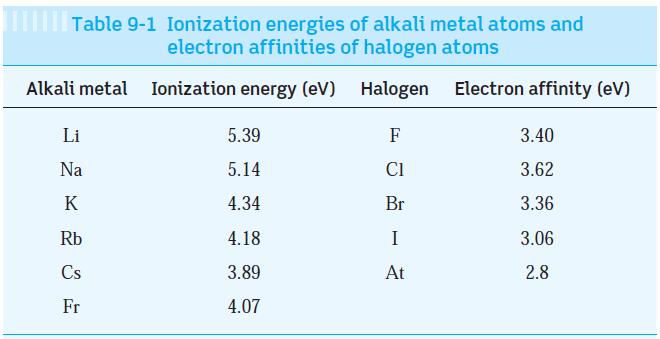
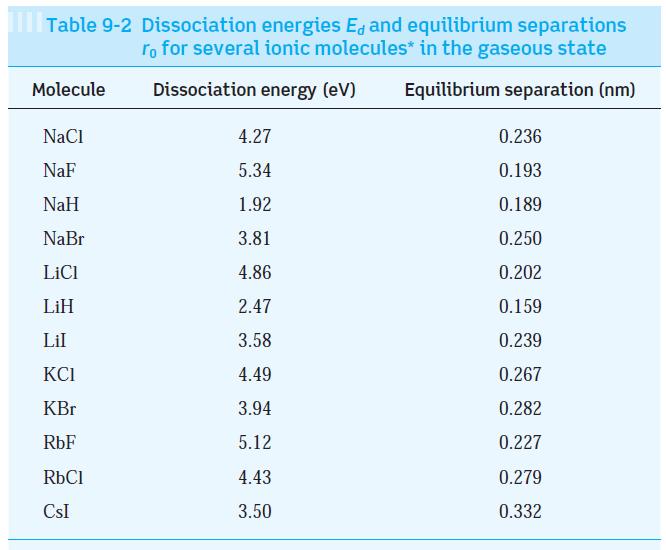
Using the data in Tables 9-1 and 9-2, estimate the dissociation energy of the three ionically bonded
Question:
Using the data in Tables 9-1 and 9-2, estimate the dissociation energy of the three ionically bonded molecules CsI, NaF, and LiI. Your results are probably all higher than those in Table 9-2. Explain why.
Tables 9-1
Tables 9-2
Transcribed Image Text:
Table 9-1 Ionization energies of alkali metal atoms and electron affinities of halogen atoms Ionization energy (eV) Halogen Electron affinity (eV) Alkali metal Li Na K Rb Cs Fr 5.39 5.14 4.34 4.18 3.89 4.07 F C1 Br I At 3.40 3.62 3.36 3.06 2.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
While E d for CsI is very close to the experimental value the ...View the full answer

Answered By

Antony Sang
I am a research and academic writer whose work is outstanding. I always have my customer's interests at heart. Time is an important factor in our day to day life so I am always time conscious. Plagiarism has never been my thing whatsoever. I give best Research Papers, Computer science and IT papers, Lab reports, Law, programming, Term papers, English and literature, History, Math, Accounting, Business Studies, Finance, Economics, Business Management, Chemistry, Biology, Physics, Anthropology, Sociology, Psychology, Nutrition, Creative Writing, Health Care, Nursing, and Articles.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Using the data in table 6-11, calculate a three-month moving average forecast for month 12.
-
Using the data in Table 116 on page 300, indicate the closing dollar value of the National City Corp. bonds that pay 4.9 percent interest and mature January 15, 2015. State your answer in terms of...
-
Using the data in Table 116 on page 300, indicate the semiannual interest payment dates for the Motorola bonds that mature in 2031. (For the item in question, look under Interest Dates.) The two...
-
Consider a two-stage compression refrigeration system operating between the pressure limits of 0.8 and 0.14 MPa. The working fluid is refrigerant-134a. The refrigerant leaves the condenser as a...
-
The following values apply for a part used in their production (purchased from external suppliers): D = 12,500 Q = 250 P = $45 C = $4.50 Required: 1. For Thomas, calculate the ordering cost, the...
-
What is the difference between signature liability and warranty liability? AppendixLO1
-
True or False. The correlation coefficient is a measure of the LO9 strength of the linear relationship between x and y.
-
Jay signed a two-year lease containing a clause that expressly prohibited subletting. After six months, Jay asked the landlord for permission to sublet the apartment for one year. The landlord...
-
Required information [ The following information applies to the questions displayed below. ] Project Y requires a $ 3 4 6 , 5 0 0 investment for new machinery with a six - year life and no salvage...
-
Tsate Kongia (birthdate 02/14/1954) is an unmarried high school principal. Tsate received the following tax documents: During the year, Tsate paid the following amounts (all of which can be...
-
Calculate the average value of the magnitude of v x from the Maxwell distribution.
-
If the exclusion-principle repulsion in Problem 9-6 is given by Equation 9-2, compute the coefficient A and the exponent n. Problem 9-6 Compute the Coulomb energy of the KBr molecule at the...
-
Gartner Systems has no debt and an equity cost of capital of 10.7%. Gartners current market capitalization is $98 million, and its free cash flows are expected to grow at 3.1% per year. Gartners...
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
What shortcomings of mechanical computation did the introduction of electronic computing devices address?
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
As an architect, you are designing a new house. A window has a height between 140 cm and 150 cm and a width between 74 cm and 70 cm. What are the smallest and largest areas that the window could be?
-
As an architect, you are designing a new house. A window has a height between 140 cm and 150 cm and a width between 74 cm and 70 cm. What are the smallest and largest areas that the window could be?
-
A 5.4-cm-diameter cylinder has a length of 12.5 cm. What is the cylinders volume in SI units?
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


