Question: For the He wave function, use with Z eff = 2 - 5/16, as obtained by the variational method. The measured value of the diamagnetic
For the He wave function, use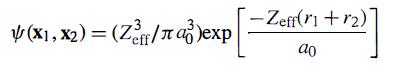
with Zeff = 2 - 5/16, as obtained by the variational method. The measured value of the diamagnetic susceptibility is 1.88 x 10-6 cm3/mole.
Using the Hamiltonian for an atomic electron in a magnetic field, determine, for a state of zero angular momentum, the energy, change to order B2 if the system is in a uniform magnetic field represented by the vector potential A = 1/2B x r.
Defining the atomic diamagnetic susceptibility x by E = -1/2X B2, calculate x for a helium atom in the ground state and compare the result with the measured value.
(X1, X2) = (Zeff/a)exp - Zerr(r+r2) ao
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
The trial wave function given in problem 519 for the helium atom is x1 x2 Zeff na expZeffr1 r2 16 where Zeff is the effective nuclear charge a is the ... View full answer

Get step-by-step solutions from verified subject matter experts


