Assign the H 2 pure rotational transitions in Figure 8.8, using data from Table 9.2 to calculate
Question:
Assign the H2 pure rotational transitions in Figure 8.8, using data from Table 9.2 to calculate the predicted frequencies. Assuming the spectra were taken at room temperature, account for the relative intensities of each transition.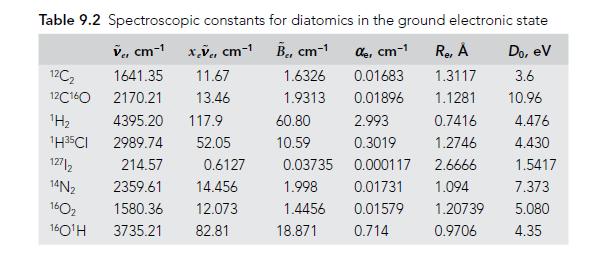
Figure 5.8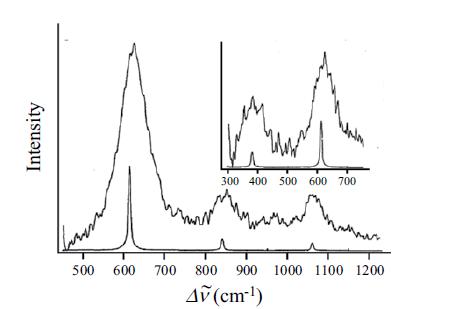
Transcribed Image Text:
Table 9.2 Spectroscopic constants for diatomics in the ground electronic state Ver cm-¹x.ve, cm-¹ B, cm-¹ de, cm-1 1.6326 0.01683 1.9313 0.01896 12C₂ 12C160 1641.35 2170.21 ¹H₂ 1H35CI 12712 14N₂ 1602 160¹H 3735.21 11.67 13.46 4395.20 117.9 2989.74 52.05 214.57 2359.61 14.456 1580.36 12.073 82.81 0.6127 60.80 10.59 0.03735 1.998 1.4456 18.871 R₂, Å 1.3117 1.1281 2.993 0.7416 0.3019 1.2746 0.000117 2.6666 0.01731 1.094 0.01579 0.714 1.20739 0.9706 Do, ev 3.6 10.96 4.476 4.430 1.5417 7.373 5.080 4.35
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Using the information from Table 92 we can assign the pure rotational transitions for H2 First we mu...View the full answer

Answered By

Allan Olal
I have vast tutoring experience of more than 8 years and my primary objective as a tutor is to ensure that a student achieves their academic goals.
4.70+
78+ Reviews
412+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.22 m glycerol (C3H8O3) in ethanol, (b) 0.240 mol of naphthalene (C10H8) in 2.45 mol of...
-
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.25 m glucose in ethanol; (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3; (c) 3.50 g NaOH...
-
Using data from Table 16.2, calculate the molar solubility of CaF2.
-
The National Health Statistics Reports dated Oct. 22, 2008, included the following information on the heights (in.) for non-Hispanic white females: a. Calculate and interpret a confidence interval at...
-
The income statement of Guesser Company is shown below. Additional information: 1. Accounts receivable decreased $63,000 during the year. 2. Inventory increased $38,000 during the year. 3. Prepaid...
-
Greenpeace Corp. wants to raise \($2.35\) million via a rights offering. The company currently has 250,000 shares of common stock outstanding that sell for \($30\) per share. Its underwriter has set...
-
Identify the four determinant attributes that set apart the Amazon, Google, and Apple smart home devices. Use those attributes to develop a compensatory purchasing model similar to the one in Exhibit...
-
1. Which of the qualities of successful entrepreneurs have Simeon and Turning Robe demonstrated? 2. Should Simeon and Turning Robe consider lowering their ingredient costs by switching to...
-
Which of the following are stockholder equity accounts?
-
Prove that the moments of inertia I a and I b (see Figure 8.3) are equal for the benzene molecule. You do not need to know the bond distances, just invoke the hexagonal symmetry, and for simplicity,...
-
The barrier to rotation about the CC bond in CH 3 CH 2 Cl is about 15 to 20 kJ/mol. Estimate the torsional frequency and predict how it could be observed experimentally. Consult a table of bond...
-
Write note on gradual loading, suddenly applied loading, and impact loading.
-
The State Public Works Division consists of the Administrator, the State Public Works Board, the Public Works Section, and the Buildings and Grounds Section. The State Public Works Board consists of...
-
Everyone knows that health care costs are high. It is also known that people tend to spend less on health care if they spend their own money, which motivated the creation of flexible spending...
-
Write a essay that critically evaluates issues in financing health care by addressing the provided prompts. Educate operational leadership on why it is important to the overall bottom line of the...
-
What are the types of conflicts that individuals may have at work? Which type have you experienced the most? 2. What are some primary causes of conflict at work? 3. Explain how miscommunication might...
-
What program do you work with that has a budget? Navy JROTC Who helps to determine how the funds are allocated and spent? US Navy and St. Elizabeth ISD How did you find out the budget amount? Does...
-
Select a piece of business mail that you received at work or at home. Analyze its appearance. What nonverbal messages does this piece send? Are these messages consistent with the content of the...
-
The manager for retail customers, Katie White, wants to hear your opinion regarding one business offer she has received from an entrepreneur who is starting a mobile phone app called Easy Money. The...
-
Identify the reagents that you would use to accomplish each of the following transformations: a. b. c. d. e. f. g. h. Br-
-
Consider the following two compounds. Monochlorination of one of these compounds produces twice as many stereoisomeric products as the other. Draw the products in each case, and identify which...
-
Draw a bond-line structure for each of the following compounds: a. CH 2 = CHCH 2 C(CH 3 ) 3 b. (CH 3 CH 2 ) 2 CHCH 2 CH 2 OH c. CH COCH 2 CH(CH 3 ) 2 d. CH 3 CH 2 OCH 2 CH 2 OCH 2 CH 3 e. (CH 3 CH 2...
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


