Question: The compound shown below has ma x = 736 nm in hexane and 553 nm in water. Explain the basis for the solvent shift.
The compound shown below has λmax = 736 nm in hexane and 553 nm in water. Explain the basis for the solvent shift.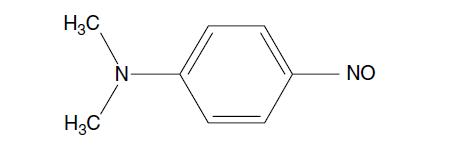
H3C H3C Z NO
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
The phenomenon youre observing where the absorption maximum max of a compound shifts depending on the solvent used is known as the solvent effect or s... View full answer

Get step-by-step solutions from verified subject matter experts


