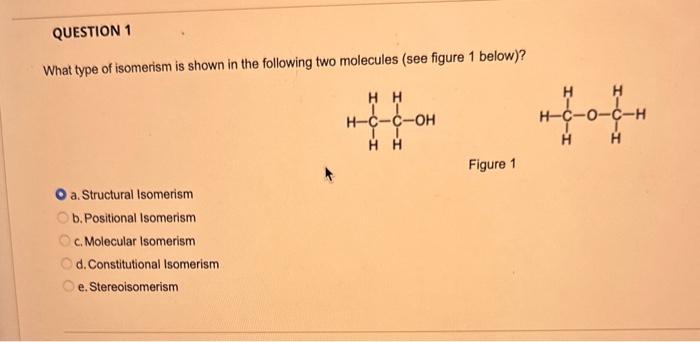
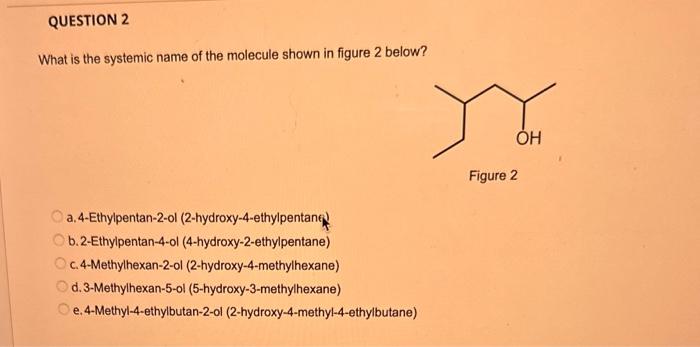
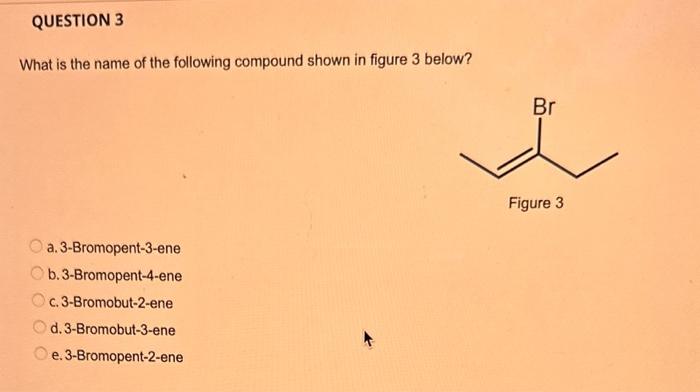
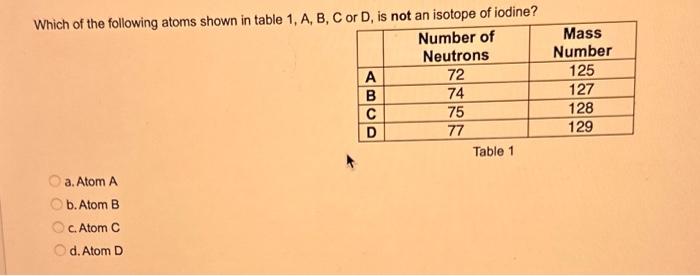
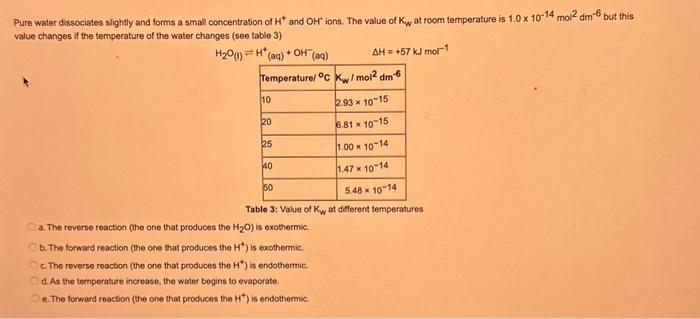
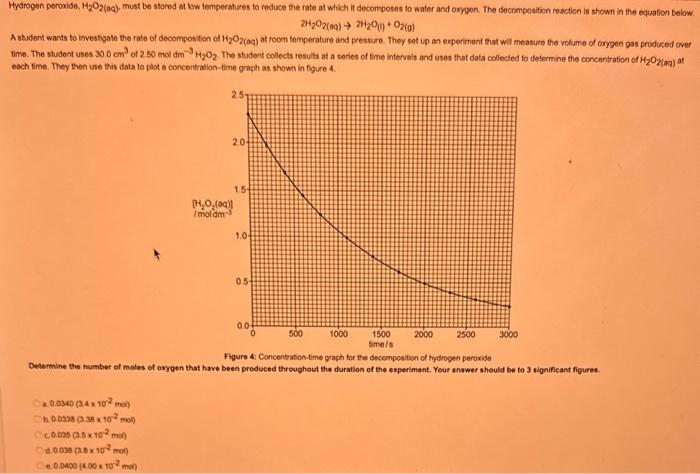
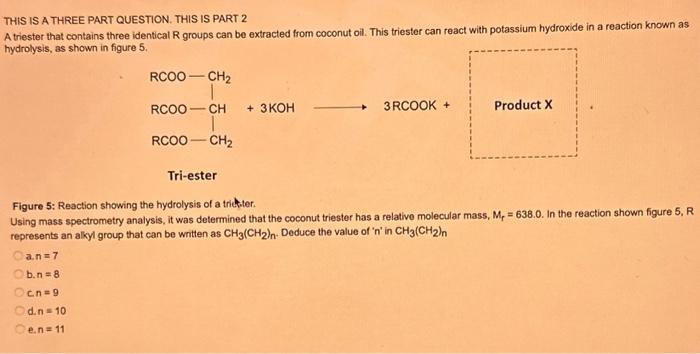
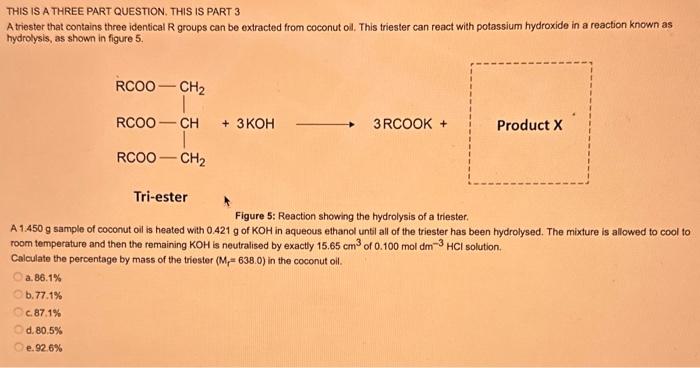
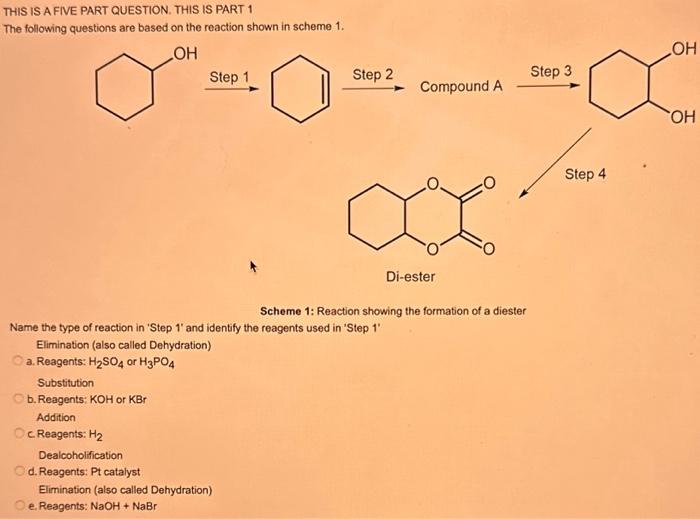
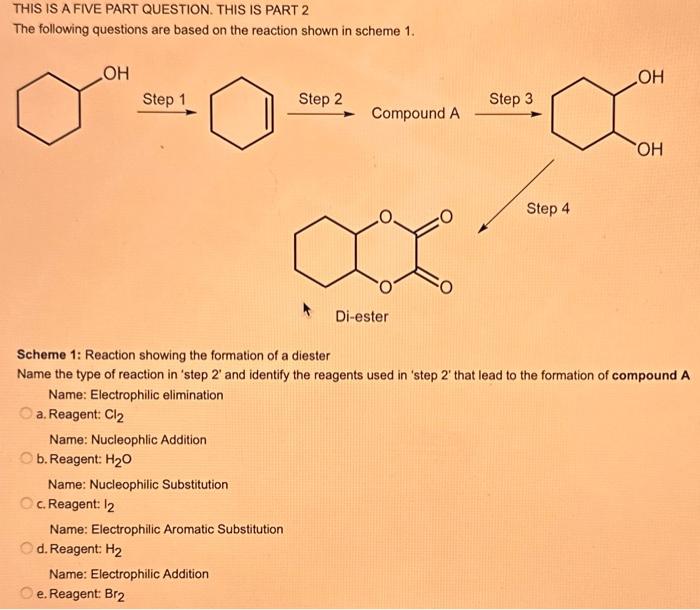
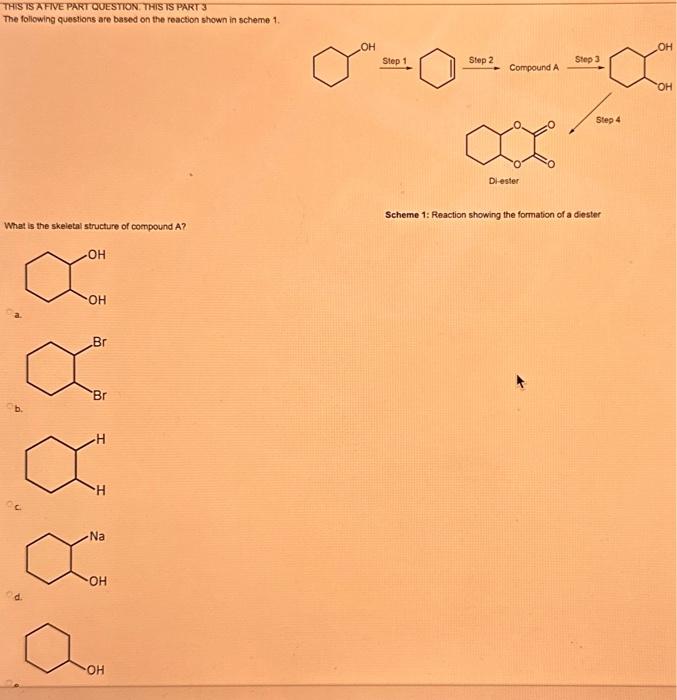
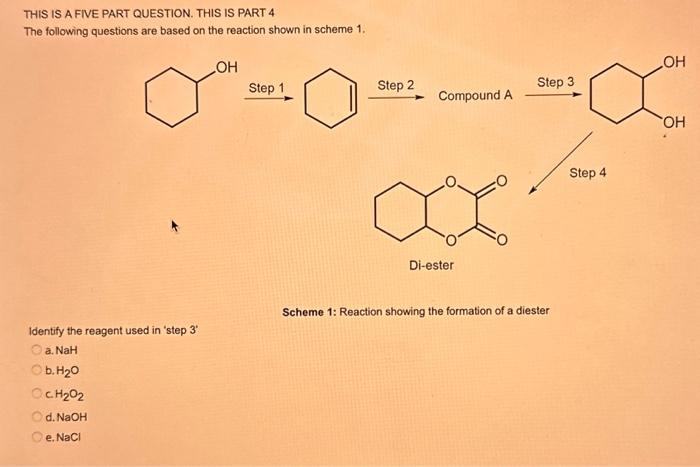
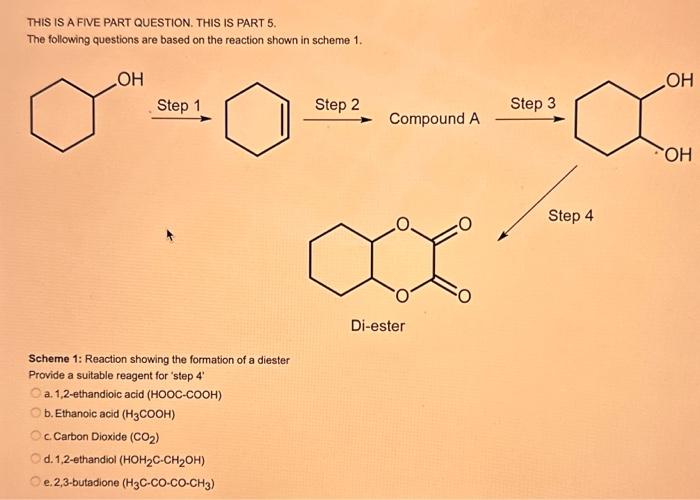
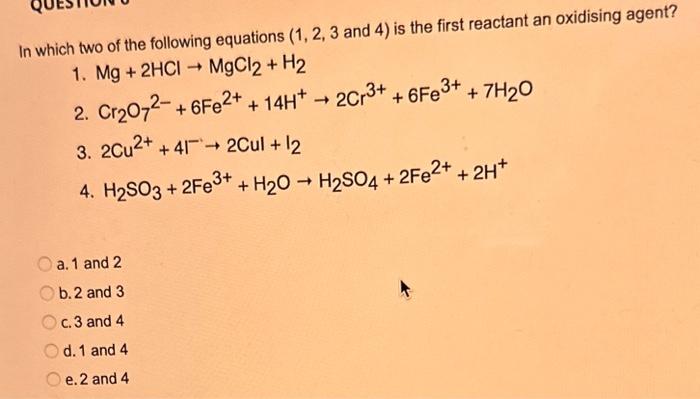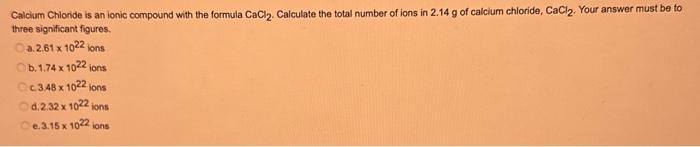What type of isomerism is shown in the following two molecules (see figure 1 below)? Figure 1 a. Structural Isomerism b. Positional Isomerism c. Molecular Isomerism d. Constitutional Isomerism e.Stereoisomerism What is the systemic name of the molecule shown in figure 2 below? Figure 2 a. 4-Ethylpentan-2-ol (2-hydroxy-4-ethylpentanff b. 2-Ethylpentan-4-ol (4-hydroxy-2-ethylpentane) c. 4-Methylhexan-2-ol (2-hydroxy-4-methylhexane) d. 3-Methylhexan-5-ol (5-hydroxy-3-methylhexane) e. 4-Methyl-4-ethylbutan-2-ol (2-hydroxy-4-methyl-4-ethylbutane) What is the name of the following compound shown in figure 3 below? a. 3-Bromopent-3-ene b. 3-Bromopent-4-ene c. 3-Bromobut-2-ene d.3-Bromobut-3-ene e. 3-Bromopent-2-ene Which of the following atoms shown in table 1, A, B, C or D. is not an isotope of iodine? Table 1 a. Atom A b. Atom B c. Atom C d. Atom D Which calcium compounds shown in table 2 contains the greatest percentage by mass of calcium? a. Calcium Carbonate b. Calcium Nitrate c. Calcium Hydroxide d. Calcium Sulfate e. Calcium Phosphate In which two of the following equations (1,2,3 and 4) is the first reactant an oxidising agent? 1. Mg+2HClMgCl2+H2 2. Cr2O72+6Fe2++14H+2Cr3++6Fe3++7H2O 3. 2Cu2++4I2Cul+I2 4. H2SO3+2Fe3++H2OH2SO4+2Fe2++2H+ a. 1 and 2 b. 2 and 3 c. 3 and 4 d. 1 and 4 e. 2 and 4 Complete combustion of 1.00g of a hydrocarbon gives 3.38g of carbon dioxide. What is the empirical formula of the hydrocarbon? a. CH b. CH2 c. CH3 d. CH4 e. C2H2 f. C2H3 g. C2H4 h. C2H5 In the first ionisation energy of neon, Ne, 1 electron is removed from each atom of Ne (g) to form Ne+(g)ions.. How many electrons are removed from 2.0210 2g of Ne(g) atoms to form Ne+(g) ions? a. 5.971023 electrons b. 5.971021 electrons c. 5.971020 electrons d. 5.971022 electrons e. 5.971019 alectrons The concentration of a solution of nitric acid is 1.0moldm3. Calculate the pH of the solution. a. 0 b. 1 c. 2 d. 3 e. 4 Find the pH of a 0.50moldm3 solution of sodium hydroxide, NaOH at 298K, given that Kw at 298Kps1.01014mol2 dm 6. a. 13.3 b. 13.4 c. 13.5 d. 13.6 e. 13.7 In a thration of sulfuric acid, H2SO4, against sodium hydroxide, NaOH,32.2mL of 0.250MNaOH is required to neutralze 26.60mL. of H2SO4. Calculate the molarity of the sulfuric acid solution. a. 0.14moldm3 b. 0.15moldm3 c. 0.16moldm3 d. 0.17moldm3 e. 0.18moldm3 Pure water dissociates slightly and forms a small concentration of H+and OHions. The value of Kw at room temperature is 1.01014mol2dmm6 but this value changes if the temperature of the water changes (see table 3) H2O(J)H+(aq)+OH(aq)H=+57kJmor1 Table 3: Value of Kw at different temperatures a. The reverse reaction (the one that produces the H2O ) is exothermic. b. The forward reaction (the one that produces the H+) is exothermic. c The reverse reaction (the one that produces the H+) is endothermic: d. As the temperature increaso, the water begins to evaporate. e. The fonward reaction (the one that produces the H+) is endothermio. THIS IS A TWO PART QUESTION. THIS IS PART 1 To investigate equilbrium a chemist mixes together sulfur dioxide, oxygen and a catalyst and compresses the gas mixture to a volume of 400cm3. The mixture is heated to a constant temperature and the reaction is allowed to reach equilibrium. The total gas volume does not change. The reaction between sulfur dioxide and oxygen to form sulfur trioxide is shown below in the balanced equation. 2SO2(g)+O2(g)2SO3(g) At equilbrium the mixture contains 0.0540molSO2 and 0.0270mol2. At the temperature used, the value for Kc is 3.045104dm4mor1. Write the expression for Kc and the units of Kc for this equilibrium. Kc=[SO3]2/[SO2]2[O2] a. Units =mol1dm3 Kc=[SO3]2[SO2]2[O2] b. Units =mol1dm3 c.Kc=[SO3]2[SO2]2/[O2]Kc=[SO3]2/[SO2]2[O2]d.Units=mol2dm6Kc=[SO2]2[O2]/[SO3]2e.Units=mor2dm3 THIS IS A TWO PART QUESTION. THIS IS PART 2 To investigate equilbrium a chemist mixes together sulfur dioxide, oxygen and a catalyst and compresses the gas mixture to a volume of 400cm3. The mixture iheated to a constant temperature and the reaction is allowed to reach equilibrium. The total gas volume does not change. The reaction between sulfur dioxide and oxygen to form sulfur trioxide is shown below in the balanced equation. 2SO2(g)+O2(g)=2SO3(g) At equilbrium the mixture contains 0.0540molSO2 and 0.0270molO2. At the temperature used, the value for Kc is 3.045104dm3mor1. Determine the amount, in mol, of SO3 in the equilibrium mixture at this temperature. YOUR ANSWER SHOULD BE TO TWO DECMINAL PLACES. a. 2.50mol b. 2.45mol c. 2.40mol d. 235mol e. 2.30mol Calcium Chionide is an ionic compound with the formula CaCl2. Calculate the total number of ions in 2.14g of calcium chloride, CaCl2. Your answer must be to three significant figures. a. 2.611022 ions b. 1.741022 ions c. 3.481022 ions d. 2.321022 ions e.3.15 1022 ions THIS IS A TWO PART QUESTION. THIS IS PART 1 The following questions will be based on the Magnesium/Copper (Mg/Cu) electrochemical cell. Write the two half-equations for the Magnesium/Copper (Mg/Cu) electrochemical cell and the shorthand representation for the Magnesium/Copper (Mg/Cu) electrochemical cell, Mg2+(aq)+1eMg+(s)Cu1+(aq)+1eCu(s)2.37Ox+0.34Red a. Mg(s)Mg2+(aq)/IlCu2+(aq)Cu(s) Mg2+(aq)+2eMg(s)Cu2+(aq)+2eCu(s)2.37Ox+0.34Red b. Cu(s)1Cu2+(aq)11Mg2+(aq)Mg(s) Mg2+(aq)+1eMg1+(s)2.37OxCu1+(aq)+2eCu1(s)+0.34Redc.Mg(s)Mg2+(aq)1/Cu2+(aq)Cu(s)Mg2+(aq)+2eMg(s)2.37OxCu2+(aq)+2eCu(s)+0.34Redd.Mg(s)Mg2+(aq)Cu2+(aq)Cu(s)Mg2+(aq)+2eMg(s)2.37RedCu2+(aq)+2eCu(s)+0.34OxeMg(s)Cu2+(aq)Mg2+(aq)Cu(s) THIS IS A TWO PART QUESTION. THIS IS PART 2 The following questions will be based on the Magnesium/Copper (Mg/Cu) electrochemical cell. Calculate the standard cell potential of the electrochemical cell Magnesium/Copper (Mg/Cu) electrochemical cell a. +2.71 b. 2.03 c. 2.71 d. +2.03 e. 2.37 Hydrogen peroxise, H2O2 (aq). must be stomed at lyw lemperatures to reduce the rate at which it decomposes fo water and oxygon. The decomposition reaction is shown in the equation below. 2H2O2(aq)2H2O(i)+O2(0) A student wants to investigate the rate of decomposition of H2O2(ag) at room temperature and pressure. They set cp an experiment that wit measure the volume of exgen gas produchd over time. The student uses 30.0cm3 of 2.50 mol dm3H2O2. The student collects resuts at a sories of time intervals and uses that data collecied to determine the concentration of H2O2(aq) at each time. They then ute this data to plot a concentration-time graph as shown in figure 4 . Figure 4: Concentration-ime graph for the decempesition of hydroget paroxide Determine the number of moles of exygen that have been produced threughout the duration of the experiment. Your answer should be to 3 significant figures. 2.0.0360(3.4102mol) b,00cosc3s4102mad c. 0.035(3.5102mot) d. 0.035.0.5102mol) e.0.0400 (4.00102max) THIS IS A THREE PART QUESTION. THIS IS PART 1 Hydrogen gas can be produced by reacting iron with a solution of hydrochloric acld. The reaction also produces iron chloride. Fe(s)+2HCl(aq)FeCl2(aq)+H2(g) A student carries out an experiment to collect the hydrogen gas produced. A0.998 g sample of pure iron is added to 30.0cm3 of 1.00moldm3 hydrochloric acid. During the experiment one of the reagents limits the amount of hydrogen produced in the reaction. Identify which reagent is the limiting one and provide the scientifio reason. Fe is the reagent that limits the hydrogen production Fe is the catalyst that is esssential for this reaction to take place and HCl is the only place that hydrogen can come from in this reaction. Without Fee, a. hydrogen would not be produced and without HCl there is no other source of hydrogen. Both Fe and HCl are the reagents that limat the hydrogen production b. Fo is the catalyst that is esssential fch this reaction to take place HCl is the reagent that limits the hydrogen production The number of moles of Fo is 0.0179 and the number of moles of HCl is 0.030. This is a 1.2 ratio, therefore, 0.179 mol of Fe would require 0.0358 mol of c. HCl to completoly react. Therefore, there is unreacted Fe remaining. d. There is no limited reagent. There is just the right number of moles of both Fe and HCl to produce the hydrogen gas. HCl is the reagent that limits the hydrogen production e. This is because it is the only place that hydrogen can come from in this reaction. THIS IS A THREE PART QUESTION. THIS IS PART 2. Hydrogen gas can be produced by reacting iron with a solution of hydrochloric acid. The reaction also produces iron chloride. Fe(s)+2HCl(aq)FeCl2(aq)+H2(g) A student camies out an experiment to collect the hydrogen gas produced. A 0.998g sample of pure iron is added to 30.0cm3 of 1.00mol3 hydrochloric acid. Calculate the mass of FeCl2 produced. a. 1.920g b.1.895g c. 1.902g d. 1.952g e. 2.002g THIS IS A THREE PART QUESTION. THIS IS PART 3 Hydrogen gas can be produced by reacting iron with a solution of hydrochloric acid. The reaction also produces iron chloride. Fe(s)+2HCl(aq)FeCl2(aq)+H2(g) A student carries out an experiment to collect the hydrogen gas produced. A 0.998g sample of pure iron is added to 30.0cm3 of 1.00moldm3 hydrochloric acid. Calculate the maximum volume, in m3, of hydrogen gas produced at 30C and 100kPa. Give your answer to 3 significant figures. a. 3.80104m3 b. 3.70104m3 c. 4.00104m3 d. 2.78104m3 e. 3.78104m3 ACOOCH/ ACOOCH+3CH+3AHCOCK+ Preduct X ACOOCH2 Trtester Flgure 5: Reaction showing the hydrelytis of a triester: THIS IS A THREE PART QUESTION. THIS IS PART 2 A triester that contains three identical R groups can be extracted from coconut oil. This triester can react with potassium hydroxide in a reaction known as hydrolysis, as shown in figure 5. +3KOH3RCOOK+ProductX Tri-ester Figure 5: Reaction showing the hydrolysis of a trittter. Using mass spectrometry analysis, it was determined that the coconut triester has a relative molecular mass, Mr=638.0. In the reaction shown figure 5 , R represents an alkyl group that can be written as CH3(CH2)n. Deduce the value of ' n ' in CH3(CH2)n a. n=7 b. n=8 cn=9 d. n=10 e. n=11 THIS IS A THREE PART QUESTION. THIS IS PART 3 A triester that contains three identical R groups can be extracted from coconut oll. This triester can react with potassium hydroxide in a reaction known as hydrolysis, as shown in figure 5. +3KOH3RCOOK+ Product X Tri-ester Figure 5: Reaction showing the hydrolysis of a triester. A 1.450g sample of coconut oil is heated with 0.421g of KOH in aqueous ethanol until all of the triester has been hydrolysed. The mixture is allowed to cool to room temperature and then the remaining KOH is neutralised by exactly 15.65cm3 of 0.100moldm3HCl solution. Calculate the percentage by mass of the triester (MT=638.0) in the coconut oil. a. 86.1% b. 77.1% c. 87.1% d. 80.5% e.92.6% THIS IS A FIVE PART QUESTION. THIS IS PART 1 The following questions are based on the reaction shown in scheme 1. Step2 Compound AStep3 Step 4 Di-ester Scheme 1: Reaction showing the formation of a diester Name the type of reaction in 'Step 1' and identify the reagents used in 'Step 1' Elimination (also called Dehydration) a. Reagents: H2SO4 or H3PO4 Substitution b. Reagents: KOH or KBr Addition c. Reagents: H2 Dealcoholfication d. Reagents: Pt catalyst Elimination (also called Dehydration) e. Reagents: NaOH+NaBr THIS IS A FIVE PART QUESTION. THIS IS PART 2 The following questions are based on the reaction shown in scheme 1. Di-ester Scheme 1: Reaction showing the formation of a diester Name the type of reaction in 'step 2 ' and identify the reagents used in 'step 2 ' that lead to the formation of compound A Name: Electrophilic elimination a. Reagent: Cl2 Name: Nucleophlic Addition b. Reagent: H2O Name: Nucleophilic Substitution c. Reagent: l2 Name: Electrophilic Aromatic Substitution d. Reagent: H2 Name: Electrophilic Addition e. Reagent: Br2 THS IS AFVVE PART QUESTION. THIS IS PART 3 The following questions are based on the reaction shown in scheme 1. step1 Stop2 Compound Astop3 What is the skeietni structure of compound A? Scheme 1: Reaction showing the formation of a dioster a. b. c: d. THIS IS A FIVE PART QUESTION. THIS IS PART 4 The following questions are based on the reaction shown in scheme 1. Di-ester Scheme 1: Reaction showing the formation of a diester Identify the reagent used in 'step 3 ' a. NaH b. H2O c. H2O2 d. NaOH e. NaCl THIS IS A FIVE PART QUESTION. THIS IS PART 5. The following questions are based on the reaction shown in scheme 1. Step1 Step2 Compound AStep3 Di-ester Scheme 1: Reaction showing the formation of a diester Provide a suitable reagent for 'step 4 ' a. 1,2-ethandioic acid (HOOC-COOH) b. Ethanoic acid (H3COOH) c. Carbon Dioxide (CO2) d. 1,2-ethandiol (HOH2CCH2OH) e. 2,3-butadione (H3CCOCOCH3)