Question: Use the data given in Table 9.2 to find the dissociation energies D e and D 0 (in units of cm1) for 2 H 35
Use the data given in Table 9.2 to find the dissociation energies D̃e and D̃0 (in units of cm−1) for 2H35Cl.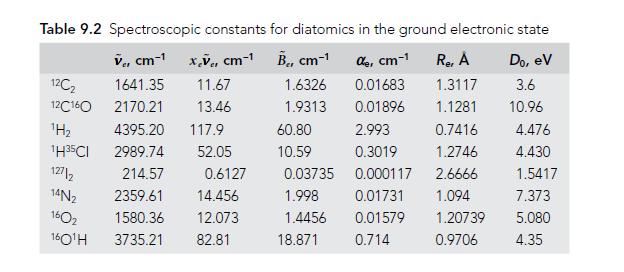
Table 9.2 Spectroscopic constants for diatomics in the ground electronic state V, cm-x,V, cm- B, cm- de, cm-1 1.6326 0.01683 1.9313 0.01896 12C 12C160 H 1H35CI 127/2 14N 160 160H 1641.35 11.67 2170.21 13.46 4395.20 117.9 2989.74 52.05 214.57 2359.61 14.456 1580.36 12.073 3735.21 82.81 0.6127 60.80 10.59 0.03735 1.998 1.4456 18.871 Re, A Do, ev 1.3117 3.6 1.1281 10.96 2.993 0.7416 0.3019 1.2746 0.000117 2.6666 0.01731 1.094 0.01579 0.714 1.20739 0.9706 4.476 4.430 1.5417 7.373 5.080 4.35
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
To find the dissociation energies De and D0 in units of cm for 2H35Cl we can ... View full answer

Get step-by-step solutions from verified subject matter experts


