Identify each of the properties illustrated in Problems 2732. a. a + (10 + b) = (a
Question:
Identify each of the properties illustrated in Problems 27–32.
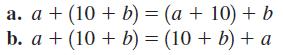
Transcribed Image Text:
a. a + (10 + b) = (a + 10) + b b. a + (10 + b) = (10 + b) + a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Ass...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify each of the properties illustrated in Problems 2732. x2+x-1 x2+x-1 x2-1 x2-1
-
Identify each of the properties illustrated in Problems 2732. 15 + [a + (-a)] = 15 + 0
-
Identify each of the properties illustrated in Problems 2732. a.mustard + catsup = catsup + mustard commutative b. (red + blue) + yellow = red + (blue + yellow)
-
A curve has equation y = x 2 ln 3x. Find the value of dy/dx and d 2 y/dx2 at the point where x = 2.
-
A glass ball of radius 2.00 cm sits at the bottom of a container of milk that has a density of 1.03 g/cm3. The normal force on the ball from the container's lower surface has magnitude 9.48 x 10-2 N....
-
List three substantive procedures the auditors could use to detect unrecorded retirements of property, plant, and equipment.
-
Tracking missiles with satellite imagery. The Space-Based Infrared System (SBIRS) uses satellite imagery to detect and track missiles (Chance, Summer 2005). The probability that an intruding object...
-
Make all required General Fund journal entries for the Doyle County Public Schools for 20X6, including adjusting entries, required by the following information. 1. The Doyle County Public Schools...
-
Periodic and Perpetual Systems and Inventory Costing Methods E7A. During July 2014, Micanopy, Inc., sold 500 units of its product Empire for $8,000 The following units were available: Cost $ 2 4...
-
Find Figure 5.18 shows the cover of a leading mathematics journal. It depicts the symbols for the twelve animals in the Chinese zodiac. (2013, for example, is the year of the snake and 2014 is the...
-
Identify each of the properties illustrated in Problems 2732. a. 5 1 = 1 5 b. 3(4 + 8) = 3 (4) + 3(8)
-
Show the converse of Theorem 2(b); i.e., show that if problem (LP1) is infeasible, then the optimum value of problem (LP2) is strictly less than zero.
-
My first run at a dissertation was on Dr. Martin Luther King, Jr. When I was very young he walked through my hometown of Albany, Georgia. My father accompanied him, more to protect him than anything,...
-
Question 2 are charged, and the charge on sphere Y is The X and Y dots shown in the figure are two identical spheres, X and Y, that are fixed in place with their centers in the plane of the page....
-
how do i get the residuel income please help in just need the cell formula in excel 2 Genmure Corporation is trying to analyze the results of three efficiency initiatives that were taken on the...
-
Harlow Appliance has just developed a new air fryer it believes will have broad market appeal. The company has performed marketing and cost studies that revealed the below information: a. New...
-
Based on the business that you created a global strategy for in the week 4 discussion, determine a low-cost & differentiation strategy in an effort to remain competitive in the global market. Include...
-
Half of the homozygous offspring survive, and one third of the heterozygous offspring survive. Suppose that the fraction of homozygous and heterozygous offspring that survive self-fertilization by a...
-
Explain why it is not wise to accept a null hypothesis.
-
Write a balanced equation for each of these cases: a) The reaction between sodium metal and bromine gas. b) The reaction between potassium metal and water. c) The reaction between hydrogen gas and...
-
Determine whether the bond between each pair of atoms will be pure covalent, polar covalent or ionic: a) S and O b) Al and F c) C and Br d) Mg and Cl
-
Write a Lewis structure for CH 3 Cl.
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


